The protein contained in the cell biofilm is called a membrane protein, which participates in and exercises a variety of cellular functions, including cell transport and information transfer, energy exchange, and the like. Membrane proteins act as receptors for a variety of neural signaling molecules, hormones and other substrates, forming channels for various ion transmembranes and constituting various transport proteins. About 30% of human proteins are membrane proteins. Most of the new FDA-approved drugs target membrane proteins. Fertilizer Spreader,Manure Fertilizer Spreader,Tractor Fertilizer Spreader,Pto Driven Fertilizer Spreader Taizhou Yingtian Agricultural Machinery Manufacturing Co., Ltd. , https://www.sakuradaagc.com
With the in-depth study of the working mechanism of membrane proteins, new targets for cell regulation have been continuously discovered, so that new drugs acting on targets can be developed more specifically. For example, there are many drugs based on cell transport channel protein design on the market. The most common type of antihypertensive drugs (such as amlodipine), its main function is calcium channel blockers, selective ion channel binding Blocking calcium ions into the cells, resulting in weakened myocardial contractility, heart rate slowdown and vasodilation to achieve antihypertensive effects. Membrane protein structure is an important basis for designing and developing drugs targeting membrane proteins, but it has been studied that the membrane protein of structure only accounts for 1% of the total membrane protein. This is because membrane proteins adhere to or span the phospholipid bilayer of the cell membrane, so purification of membrane proteins, analysis of structures, and the like are extremely challenging.
The analysis and study of proteins including membrane protein structures is the basis of protein research. X-ray crystallography, nuclear magnetic resonance (NMR) and cryo-electron microscopy (Cryo-EM) are called the three major tools of structural biology research. More than 90% of the membrane protein structures use X-ray crystallography, and NMR plays an important role in the analysis of small molecular weight proteins. X-ray crystallography has been the preferred method of analyzing protein structures for decades. However, the X-ray crystallography method is very demanding on the sample, and it is necessary to obtain a protein crystal having diffraction ability. But many proteins, especially membrane proteins and protein complexes, are difficult to crystallize. On the other hand, NMR can be used to resolve the structure of smaller proteins (generally no more than 30 kDa), but it is more difficult to use for structural analysis of larger molecular weight proteins. For a long time before, cryo-electron microscopy has not been widely used due to its low resolution resolution structure. In recent years, breakthroughs in hardware and algorithms in the field of cryo-electron microscopy have enabled the use of cryo-EM to resolve near-atomic resolution structures and the development of structural biology.
Unlike crystallography, cryo-EM requires very little sample, no crystal formation, and only needs to be stable in an aqueous environment. At the end of 2013, Professor Cheng Yifan of the University of California, San Francisco, collaborated with colleague David Julius to identify a membrane protein that plays a central role in pain and heat perception with near-atomic resolution (3.4 Ã…) using a single-electron counting detector. The structure of TRPV1. This research has enabled researchers in related fields to re-examine the role of cryo-electron microscopy in structural biology research. This provides a new opportunity for the study of structural biology of some proteins that are difficult to crystallize, especially membrane proteins. With the continuous experiment, the results of the analysis of membrane protein structure by cryo-electron microscopy are more and more. As an application in drug development, it is necessary to analyze the interaction between small molecules and proteins, and the higher the resolution, the better. Subramaniam et al. now achieve the highest resolution (2.2 Ã…) of cryo-EM imaging to date, after only X-ray crystal diffraction has reached this level of resolution, which provides enough structural information for better Drug Discovery. 
Figure 1: Effect of resolution on observations (left) Molecular structure that can be determined for different resolutions (right)
(Source: Elad Binshtein, Melanie D. Biochemistry, 2015)
Membrane proteins can be divided into three major categories: integral membrane proteins, peripheral membrane proteins, and lipid-anchored proteins. The integral membrane protein accounts for 70% to 80% of the membrane protein species. The peripheral membrane proteins are generally water-soluble, relatively easy to separate and purify, and are also relatively easy to obtain crystals for X-ray diffraction analysis. It is difficult to study the integrated membrane protein. Because of its hydrophobic transmembrane region, it is very unstable to be directly exposed to an aqueous solution environment, so it is necessary to add a detergent to stabilize. When detergents are used to protect membrane proteins for structural analysis and functional studies, membrane proteins are lost from their natural phospholipid bimolecules and lose important information about lipid interactions and their effects on protein structure. This has a particular impact on proteins that require lipid structure and regulation. In addition, inappropriate detergents can destabilize membrane proteins, and structural differences between detergent micelles and phospholipid molecules and water-fat interface curvature may, in some cases, result in changes in membrane protein structure.
In order to better stabilize the membrane protein in the aqueous environment by cryo-electron microscopy and avoid the potential effects of detergents, Nanodisc, which simulates the phospholipid bilayer structure of cell membranes, guarantees that the experimental results are not subject to various A solution to the effects of uncertainties. Nanodisc was first proposed by Professor Stephen Sligar of UIUC, a phospholipid bilayer membrane structure composed of membrane scaffold proteins (MSPs) and phospholipid molecules. The physicochemical properties of Nanodisc are similar to those of cell membrane phospholipid bilayers, and membrane proteins can be integrated into Nanodisc to maximize their structural stability and biological activity, providing powerful technical support for membrane protein research. Membrane scaffold proteins (MSPs) are reduced versions of apolipoprotein (apo) AI that surround the lipid bilayer to form a disc-like structure, Nanodisc. It contains a hydrophobic face facing the inner lipid layer and a hydrophilic face facing outward. This structure allows Nanodisc to have high solubility in aqueous solution with very high stability. Through the Nanodisc package, the purified membrane protein will maintain the spatial structure stability and activity, and will also simulate the state of the membrane protein on the cell membrane. The transmembrane domain is embedded in the phospholipid bilayer. 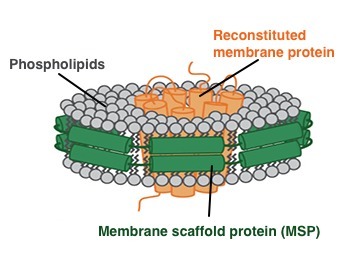
Figure 2: Schematic diagram of membrane protein assembly to Nanodiscs.
Green: Membrane Scaffold Proteins (MSPs); Gray: Phospholipids; Orange: Membrane Protein (Source: Cube Biotech Official Website)
There are two main methods for assembling membrane proteins into Nanodiscs. 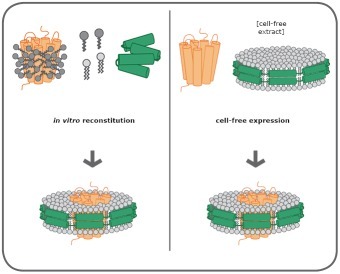
Figure 3: Two assembly mechanisms for membrane proteins and Nanodisc (Source: Cube Biotech Official Website)
Left panel: Membrane protein (orange) is dissolved in detergent (dark grey) and mixed with lyophilized MSP (green) and phospholipids (light gray). The detergent is then removed to form a protein-Nanodisc complex.
Right: Nanodisc pre-assembled with MSP and phospholipids is added to cell-free expression systems, and the new membrane proteins are spontaneously assembled into Nanodisc.
Combining the two technologies of Nanodisc and cryo-electron microscopy, more and more membrane protein structures are resolved. Among them, Professor Cheng Yifan revealed the mechanism of action of ligands and lipids by elucidating the structure of TRPA1 in Nanodisc, and determined the location of cyclic lipids and lipids, confirming the formation of a ternary complex. Specific phospholipid interactions promoted the binding of a related ligand such as spider toxin to TRPV1, and the results were published in 2016 Nature. In addition, because Nanodisc can also achieve oligo, dimerization and monomer mosaic on the same Nanodisc, Rouslan G and its collaborators from Europe have embedded ryanodine receptor tetramers on Nanodisc to achieve structural analysis using cryo-electron microscopy. Related results were also published in the 2015 Nature Journal. It is believed that with the continuous improvement of the analytical ability of cryo-electron microscopy and the continuous emergence of natural environmental technologies such as Nanodisc stable membrane protein and reduced membrane protein, membrane protein structure analysis and functional research will usher in a new development. 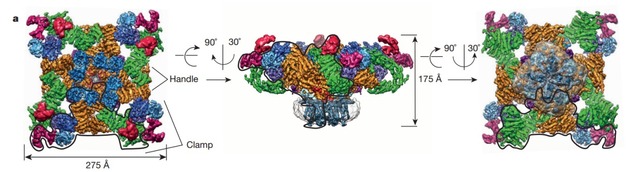
Figure 4: Analysis of the structure of the TRPA1 protein by Nanodisc by cryo-electron microscopy, the gray part of b, c is Nanodisc
(Image courtesy of Cheng Yifan 2016 Nature article) 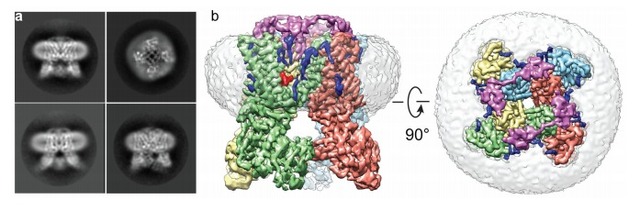
Figure 5: Analysis of Nanodis-wrapped ryanodine receptor tetramer structure by cryo-electron microscopy, gray part is Nanodisc
(Image courtesy of Rouslan G 2015 Nature article)
reference:
1. Yifan Cheng, et al. A Primer to Single-Particle Cryo-Electron Microscopy. 161(3): 438 (2015).
2. Elad Binshtein, Melanie D. Ohi Cryo-Electron Microscopy and the Amazing Race to Atomic Resolution [J]. Biochemistry, 54: 3133-3141 (2015).
3. Yifan Cheng, et al. Single-particle electron cryomicroscopy. Nature Methods 11, 30 (2014).
4. Li, X. et al. Electron counting and beam-induced motion correction enablenear-atomic-resolution single-particle cryo-EM. Nature Methods 10, 584–590 (2013)
5. Bayburt, TH, Grinkova, YV & Sligar, SG Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Lett. 2, 853–856 (2002).
6. Bartesaghi A, Merk A, Banerjee S, Matthies D, Wu X, Milne JL, Subramaniam S. Electron microscopy. 2.2 Å resolution cryo-EM structure of β-galactosidase in complex with a cell-permeant inhibitor. Science. 5; 348 (6239): 1147-51 (2015).
7. Sun L, Zhang X, Gao S, Rao PA, Padilla-Sanchez V, Chen Z, Sun S, Xiang Y, Subramaniam S, Rao VB, Rossmann MG. Cryo-EM structure of the bacteriophage T4 portal protein assembly at near -atomic resolution. Nat Commun. 6;6:7548 (2015).
8. Ilia G Denisov, Sligar, SG Nanodiscs for structural and functional studies of membrane proteins. Nature Structural & Molecular Biology 23, 481–486 (2016)
9. Rouslan G. E, et al. Architecture and conformational switchmechanism of the ryanodine receptor. Nature 517. 39 (2015).
10. Lagers tröm, MC et al. Structural diversity of Gprotein-coupled receptors and significance for drug discovery. Nature Reviews Drug Discovery 7, 339-357 (2008).
11. Hagn, F. et al. Optimized phospholipid bilayer Nanodiscs facilitate high-resolution structure determination of membrane proteins. J. Am. Chem. Soc., 135: 1919-1925 (2013).
12. Wang, Z. et al. Tyrosine phosphorylation of Mig6 reduces its inhibition of the epidermal growth factor receptor. ACS Chem. Biol. 8(11): 2372-6, (2013).
13. Timothy H. B, et al. Transducin Activation by Nanoscale Lipid Bilayers ContainingOne and Two Rhodopsins. JOURNAL OF BIOLOGICAL CHEMISTRY. 282 (2007).
14. Krueger-K, et al. An evaluation of detergents for NMR structural studies of membrane Proteion. J Biomol NMR, 28(1) 43-57 (2004).
15. Yoshiura C, et al. NMR analyses of the interaction between CCR5 and its ligand using functional reconstitution of CCR5 in lipid bilayers. J Am Chem Soc, 132 (19): 6 768-6 777 (2010).
16. Erik Henrich, et al. Analyzing native membrane protein assembly in nanodiscs by combined noncovalent mass spectrometry and syntheticbiology. eLife6:e20954 (2017).
17. Moers et al., Modified lipid and protein dynamics in Nanodiscs. Biochim. Biophys. Acta, 1828(4): 1222-9, (2013).
18. Nasr. et al., Radioligand binding to Nanodisc-reconstituted membrane transporters assessed by the scintillation proximity assay. Biochemistry, 14;53(1):4-6, (2014).
19. Bi Yunchen, Wang Yujuan, Wang Junfeng. Application of Nanodisc System in the Study of Structure and Function of Membrane Proteins. Journal of Spectroscopy, 28(2): 177-189 (2011)
20. Zhang Kai. What are the new technologies that are most concerned by the scientific community in 2015? Know the column, (2016)
21. Dr. Cheng Yifan's Nature published breakthrough results. Bio-Communication, (2016)
22. Nature Methods Announces 2015 Technology of the Year. Bio-Communication, (2016)