The accuracy and precision of pipetting not only requires matching of the tip and the pipette, but also a tip suitable for liquids. When a standard tip (PP) is used to remove a liquid having a small surface tension, it is easy to leave a film (such as a liquid containing a detergent) on the surface of the tip. Residues of the liquid can result in inaccuracies and inconsistencies in the pipetting results, as well as loss of some expensive samples. The reagents and samples used in many DNA and protein assays typically contain a detergent. The development of low adsorption tips is a common problem in order to improve liquid residue. Different manufacturers use different techniques to make low-adsorption tips, so they are different in consistency, hydrophobicity, and chemical resistance. The following is a comparison of the chemical resistance of the low adsorption tips of various manufacturers. material Absorbance test in conclusion: Web:
operation in China.
Intranasal spray administration, no injection and no pain
Produced with SPF embryonated eggs
pollution is excluded.
Influenza Vaccine,Influenza Vaccine Development,Influenza Vaccine Strains,Flu Vaccine Changchun BCHT Biotechnology Co. , https://www.ccbcht.net
Sartorius Optifit Standard Tip: 1000μ
Sartorius Optifit Low Adsorption Tip: 1000μl
Other manufacturers low adsorption tips: 1000μl
Sartorius Picus electric pipette: 50-1000μl, aspirate at 4 speeds, 1 speed discharge chemical reagent: isopropanol, acetonitrile, DMF (dimethylformamide), green food dye
Low adsorption pipette tips from 3 other manufacturers
96-well microplate spectrophotometer (Biotek)
In the absorbance test, we used a staining reagent (green food dye dissolved in distilled water) to determine the residual liquid in the pipette tip after draining. Use the largest nominal volume of the measured tip to absorb the green reagent. The liquid is then drained directly back into the container. Next, rinse the tip 5 times with distilled water at the maximum capacity of the tip. The absorbance of the rinse was then measured using a spectrophotometer (405 nm) and the results were compared to a reference reagent. The absorbance of the rinse is directly related to the amount of liquid remaining in the tip.
Chemical resistance test
1000 μl Solvent: Repeat the pipetting of isopropanol, acetonitrile and dimethylformamide 20 times using a 1000 μl pipette tip. The tip was rinsed 3 times with distilled water. The effect of this treatment on the performance of the low adsorption tip was then analyzed using an absorbance test using a dyed liquid as a reagent. Repeat 6 tests for each of the 6 tips. The results of the chemically treated tips were compared to the results of the untreated standard and low adsorption tips.
Chemical resistance of low adsorption pipette tips <br> A variety of techniques have been used to create low adsorption surfaces for pipette tips. The most stable method produces a completely hydrophobic and non-leaching tip. As shown in Figure 1, there is a significant difference in chemical resistance between the low adsorption tips tested. The low adsorption function of some manufacturers' tips is significantly reduced after treatment with selected solvents. The performance of Sartorius low-adsorption tips after chemical testing was at the same level as without any chemical treatment, indicating that these tips were inert and leached. Autoclaving the Sartorius low-adsorption tips also did not affect the performance of the tips (data not shown). 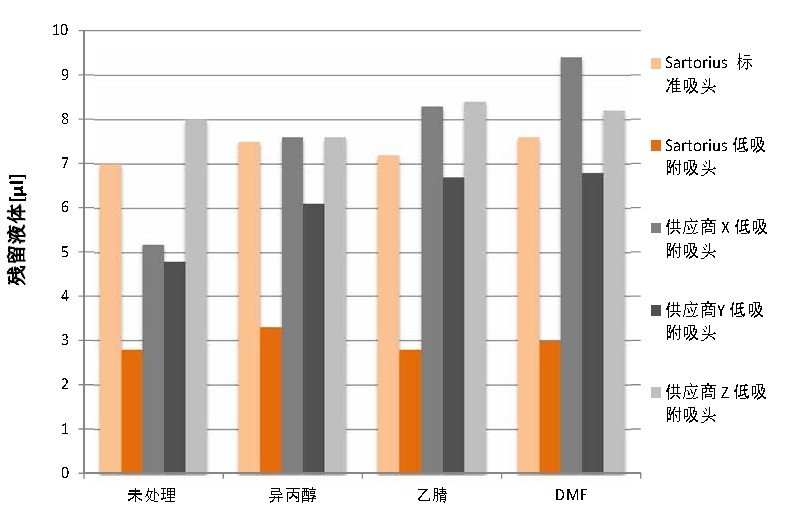
Figure 1 Comparison of chemical resistance of low adsorption pipette tips
Compare the low adsorption tips of the three suppliers with Sartorius standard and low adsorption tips. The chemical resistance test was performed according to the absorbance test method using a Sartorius Picus electric pipette (1000 μl) and a 1000 μl pipette tip. Repeat the test for each of the 6 tips of each supplier.
There are significant differences in chemical resistance between the various low adsorption tips on the market. The Sartorius low-adsorption tip has the best chemical resistance, ensuring low adsorption properties without the influence of liquids and without leaching.
Tel: 400.920.9889 | 800.820.9889
Original link
University question on small tips (one) - chemical resistance
Influenza Vaccine, Live, Nasal, Freeze-dried Exclusively authorized by the WHO in China WHO
Influenza Vaccine, Live, Nasal, Freeze-dried is a cooperative project with the WHO, which has been included in Global Action Plan for Influenza Vaccines (GAP)[1]. Hundreds of millions of doses have been used in the world, and Changchun BCHT Biotechnology Co. has the exclusive right of production and
The vaccine is inoculated by nasal spray, equipped with nasal spray device, and only needs to spray once a year in two nostrils to prevent influenza.
Mucosal immunity + Cellular immunity + Humoral immunity
After influenza virus attacks human body, it widely exists in nasal cavity, respiratory tract and other mucosal parts, as well as in body fluids and cells.
Vaccination of Influenza Vaccine, Live, Nasal, Freeze-dried can quickly stimulate the triple immune response of human body, and carry out defense
against viruses in different parts:
·Intranasal administration can produce mucosal immunity (IgA antibody), which forms the first immune defense line in the nasal cavity.
·Produce humoral immunity (IgG antibody) to remove influenza virus from body fluids.
·Produce cellular immunity (T cells) to remove influenza virus from cells
3+N More extensive protection
The production strains are recommended and supplied by the WHO every year.
The vaccine can not only effectively resist the vaccine strain, but also produce cross immunity to other subtypes of influenza virus.
The chick embryos for vaccine production comes from SPF (specific pathogen free) chicken flocks, so the risk of exogenous pathogenic microorganism
Free of inactivator, split agent and preservative
High protection and fast antibody production
This is the first influenza vaccine in China that has been evaluated by the protection effect of etiology in clinical stage. The protective effect of phase III
clinical trial is consistent with the literature[2]
.
First, the immune barrier was formed in the nasal mucosa after LAIV vaccination. It has been reported that the antibody of nasal mucosa can be
produced in 3 days[3], which greatly shortens the time for vaccine to produce
protective effect.