Scoop Stretcher,Scoop Style Stretcher,Plastic Scoop Stretcher,Ambulance Scoop Stretcher jiangyin chenyi medical technology co.,ltd , https://www.chenyimed.com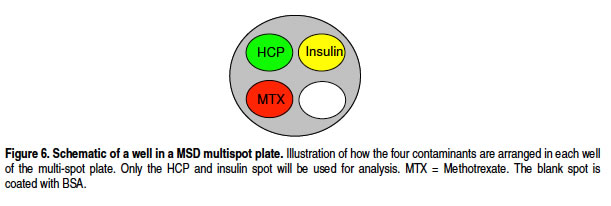
MSD Electrochemiluminescence Technology for Quantification of Impurities in Biological Processes
[Biopharmaceutical production quality control department, biopharmaceutical CMO enterprise] Rare drug factory uses ECL technology to quantify impurities in biotechnology
Implementation of electrochemiluminescence technology for quantification of bioprocess impurities
Quantification of impurities in biological processes using electrochemiluminescence technology ECL
Published: 2008
Author: Uppsala University (Uppsala universitet in Swedish), ranked first in Sweden, 21st in Europe and 71st in the world.
Experimental unit: Swedish Orphan Biovitrum (Sobi), with a market capitalization of $4.3 billion. In 2015, Pfizer intended to acquire the Swedish pharmaceutical company that develops drugs for the treatment of rare diseases. It mainly develops rare diseases, including leukemia, autoimmune diseases, metabolic diseases, and cancer support therapies. Mainly distributed in Europe, North America, Australia, New Zealand. The company develops in the field of specialty medicines, including inflammation/immune diseases, lysosomal storage diseases, blood diseases, obesity, and protein replacement therapy.
On January 8, 2013, Swedish Orphan Biovitrum (Sobi) announced that the US Food and Drug Administration (FDA) has approved Kineret (anakinra, anakinra) for early-onset multisystem inflammatory disease in children and adults. Disease (NOMID) treatment. This product is the first and only FDA-approved NOMID treatment, and NOMID is the most severe symptom of cryopyrin protein-related periodic syndrome (CAPS). This is the first time this product has been approved for use in children. It has also been approved by the FDA for orphan drugs. There is currently no suitable therapy for patients with NOMID, and this product has been granted priority review based on its potential for efficacy. As early as 2001, this product was approved for the treatment of human rheumatoid arthritis (RA).
CAPS is a disease that is lifelong and causes severe debilitation in the body. NOMID is the most serious symptom in this category and is associated with excessive secretion of interleukin-1 (IL-1) immune system proteins. As the disease progresses, the patient's hearing and vision gradually decline, cognitive function is impaired, and different degrees of joint deformation are caused. The drug's five-year study shows that in addition to controlling daily symptoms such as fever, rash, headache, joint pain, etc., this product has a definite effect on stabilizing the functions of the central nervous system (CNS) such as hearing and vision. .
Electrochemiluminescence technology: from Meso Scale Discovery, USA.
Research purposes: Use MSD ECL instead of ELISA to make detection faster and better.
In recombinant protein manufacturing for the purpose of producing protein-based drugs it is important to be able to demonstrate that impurities have been removed after the purification process and that the final product is pure. This project is involved in quantification of some of the impurities That may be found in a biopharmaceutical product; insulin, insulin like growth factor 1 (IGF-1) and host cell proteins (HCP). Analysis techniques that are to be used have to meet high demands in both sensitivity and in precision. Today the Process related impurities are mainly analyzed with enzyme linked immunosorbent assays (ELISAs) that are available commercial as kits. However a new technique developed by Meso Scale Discovery (MSD) where the detection is based on electrochemiluminescence (ECL) technology is starting to replace ELISA. The reason why the ECL assay may be superior to ELISA is that the measuring range is wider and the detection limit of the technique often is mu Ching lower, which means that the sensitivity is increased. Also the possibility to test multiple analytes in the same assay, a multiplex assay, reduces time and work load. This project evaluates this new technology by comparing results from the ECL assay with ELISA. Initially With one analyte at the time and if this comparison is successful the assays will be tested in a multiplex ECL assay format.
in conclusion:
• The ECL assay is suitable for insulin and more sensitive than the ELISA currently used.
MSD electrochemiluminescence technology is more suitable for insulin detection than ELISA, and its sensitivity is higher than the ELISA used.
• The singleplex and duplex assay for HCP generate similar signals.
Host cell protein HCP single-factor detection and multi-factor detection signals are consistent (using MSD multi-factor technology can simultaneously detect host cell protein HCP and drugs in one well, saving time and labor)
The full text of the paper can be requested by replying to the email and contacting the email address.