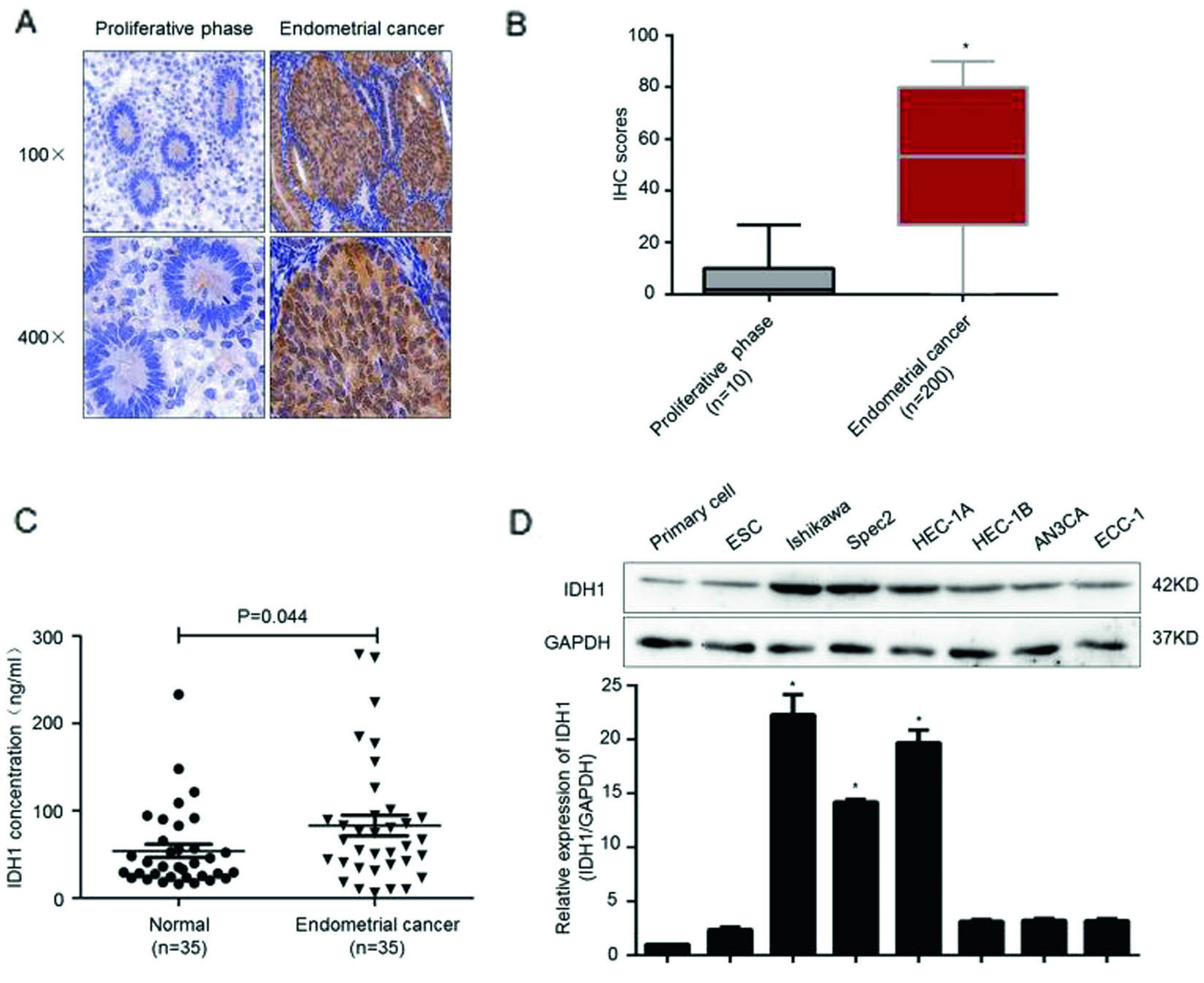
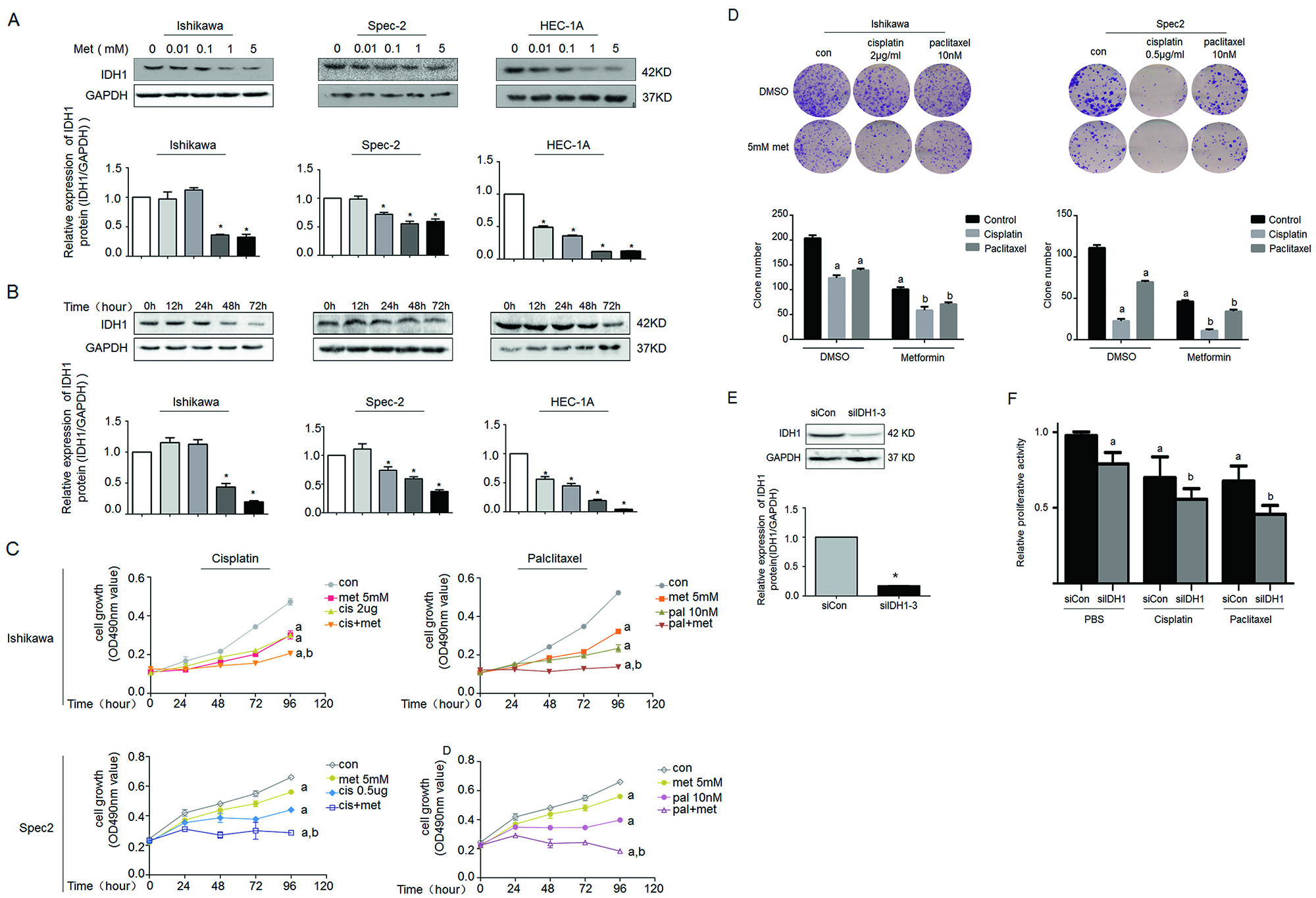
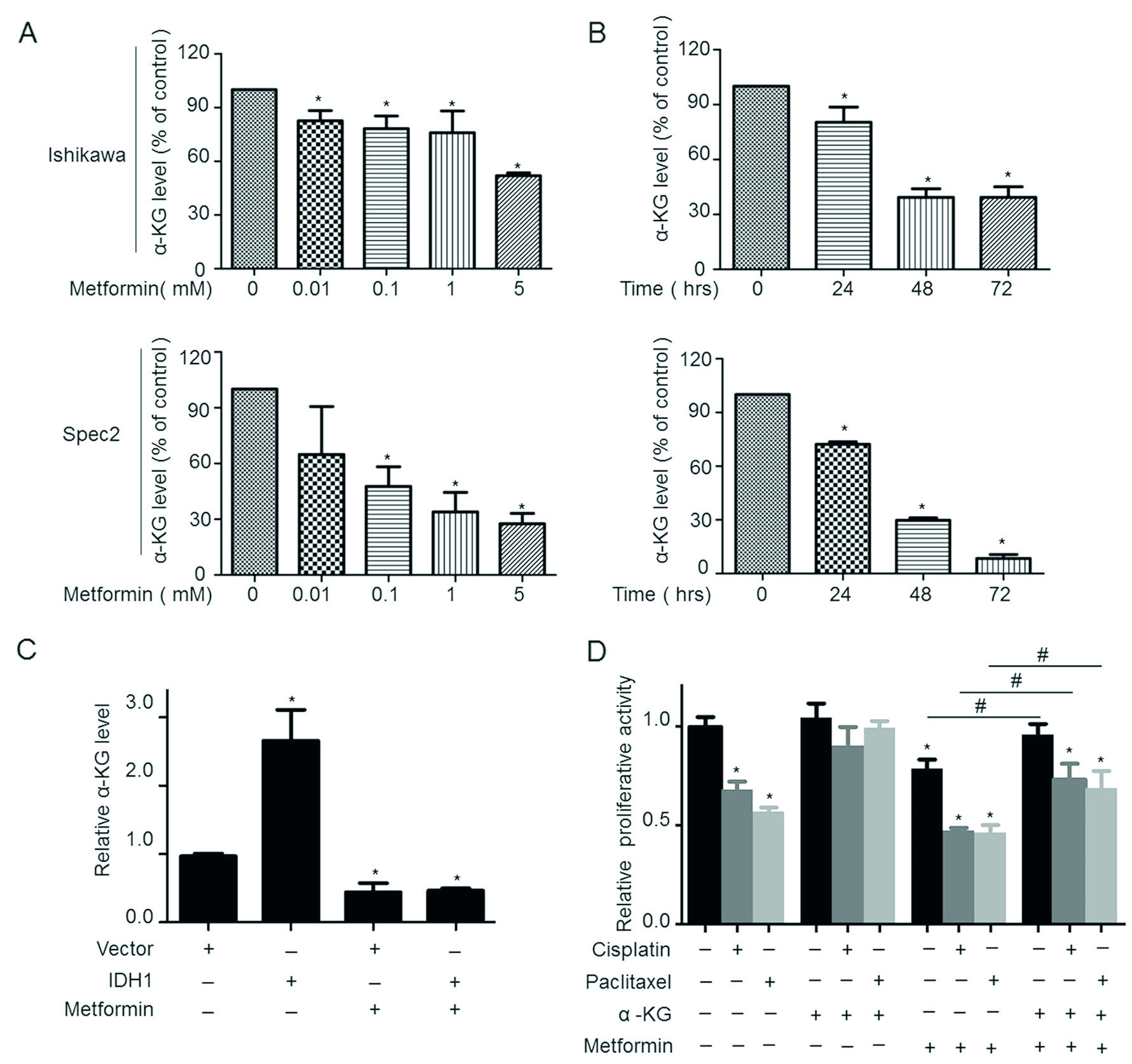
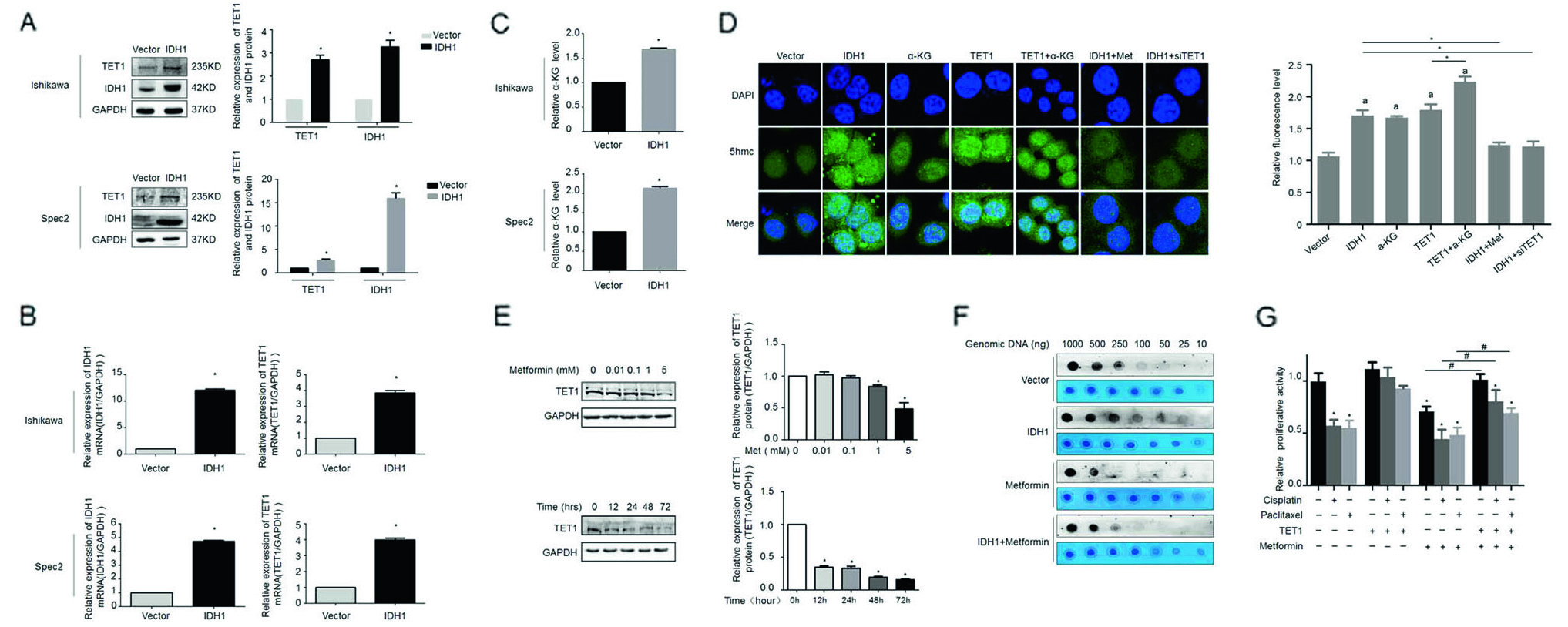
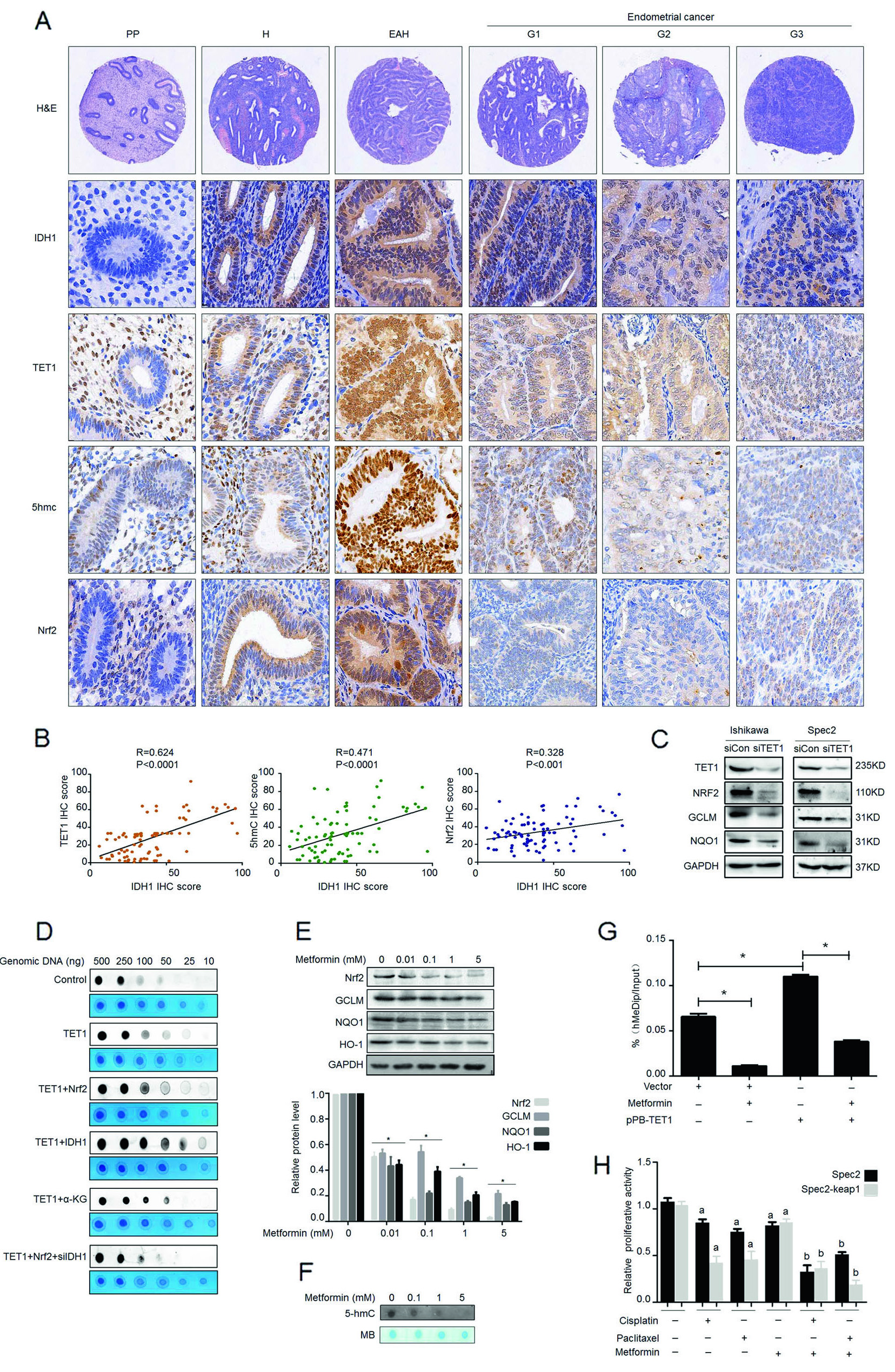
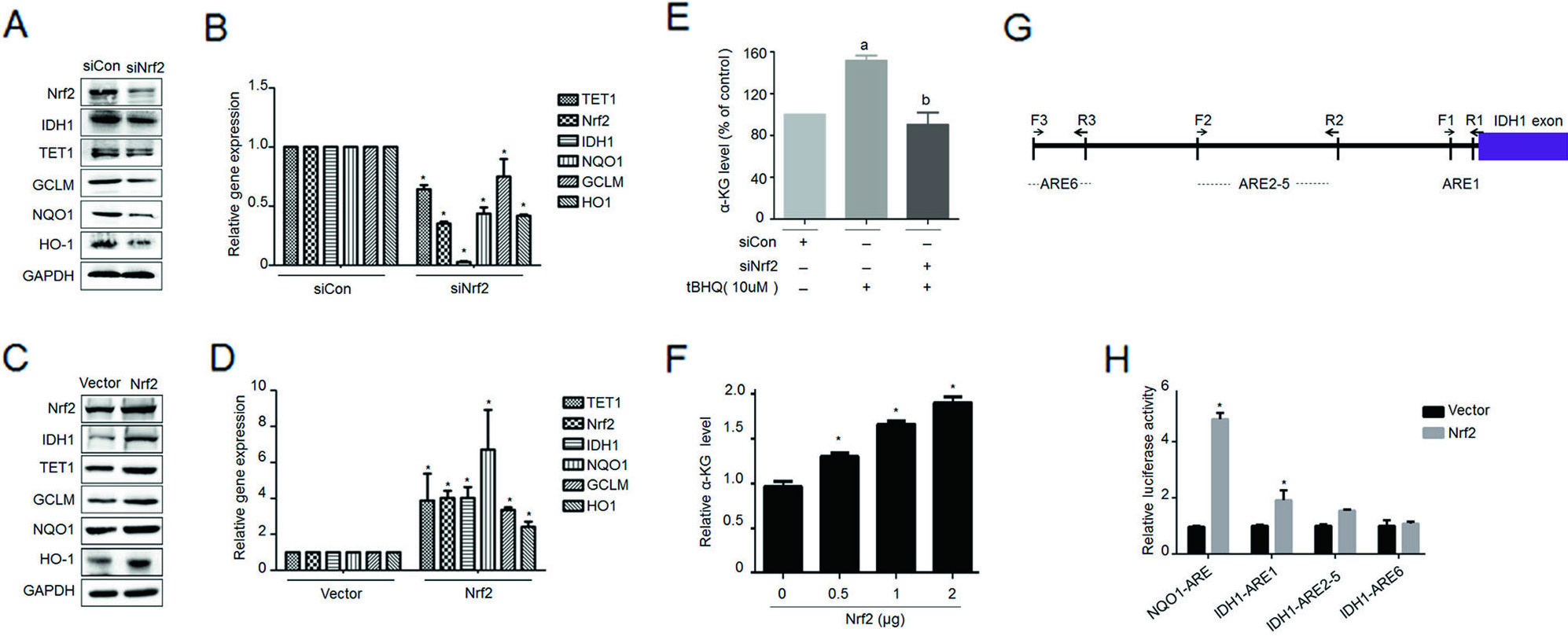
The Zhang Yibo team of the First People's Hospital Affiliated to Shanghai Jiaotong University was published in the Nature sub-publishing Oncogene ( influence factor: 6.854 ) under the title "Metformin sensitizes endometrial cancer cells to chemotherapy through IDH1-induced Nrf2 expression via an epigenetic mechanism" . This study, for the first time, reveals a new mechanism for the inhibition of endometrial cancer by "magic drug" metformin, pointing out the key role of DNA methylolation modification in this process. Research Background: Metformin, because of its strong hypoglycemic ability and small side effects, is the main sugar-control drug for diabetic patients. With the deepening of research, other biological functions of metformin have gradually been discovered. Relevant research has appeared frequently in journals such as Nature, Cell, and Cell Stem Cell, including: tumor suppression, blood fat reduction, blood pressure lowering, fatty liver improvement, and improvement. Multiple ovarian syndrome, fat loss, life extension and prevention of Alzheimer's disease, etc.! ! Therefore, it was called the title of “God Medicineâ€. DNA methylolation, known as the "sixth base" in mammalian DNA, is closely related to major human diseases such as cancer and neurodegenerative diseases, and is a new hot spot in the field of epigenetics. The authors of this article detected DNA methylolation modifications in endometrial cancer cells ( DNA methylation-related services, provided by cloud-sequence organisms ) and demonstrated that this modification promotes cancer cell proliferation. Subsequently, the authors treated the proliferating cancer cells with "God medicine" and other anticancer drugs and found that the proliferation process was inhibited. What exactly did the author do? Let's take a closer look. research sample: Human cell line: endometrial cancer cell line (Ishikawa, HEC-1A, SPEC2, HEC-1B, AN3CA, ECC-1), normal endometrial cell line. Human tissues: 200 cases of endometrial cancer tissues (160 cases of unstaged samples; 40 cases of staged samples: 25 cases of G1 stage, 8 cases of G2 stage, 7 cases of G3 stage) and 10 cases of proliferative endometrial tissue. Research result: 1.IDH1 is highly expressed in endometrial cancer tissues and cancerous cell lines By immunohistochemistry, the authors found that the positive staining rate of IDH1 gene in cancerous endometrium was significantly higher than that in proliferative endometrial tissue (Fig. 1A, B). In order to ensure accurate results, the authors expanded the sample to 35 people in each group. The expression of IDH1 in plasma was detected by ELISA. The expression of IDH1 in serum of cancer patients was also confirmed (Fig. 1C). So, what is the expression of the IDH1 gene in cells? By detecting 8 seed endometrial cancer cell lines (Fig. 1D), it was detected at the mRNA and protein levels that the IDH1 gene was highly expressed in the Ishikawa, Spec2, HEC-1A and HEC-1B cell lines. In summary, IDH1 is highly expressed in endometrial cancer tissues and cancerous cell lines. Figure 1. Expression of IDH1 in tissues and cell lines 2. Metformin enhances the sensitivity of endometrial cancer cells to chemotherapy by inhibiting the expression of IDH1 The authors used the cell line expressing the top 3 gene of IDH1 gene as a material to detect the amount of metformin and the expression of IDH1 in the reaction time. The results confirmed that metformin inhibited the expression of IDH1 (Fig. 2A, B). In addition, cell proliferation and counting experiments confirmed that metformin and anticancer drugs synergistically inhibit cancer cell proliferation (Fig. 2C, D). Can IDH1 participate in the above cancer cell proliferation process? The authors successfully inhibited IDH1 gene expression by in vitro interference experiments (Fig. 2E), and detected that the interfering cells had lower proliferative capacity than the control group after treatment of the two anticancer drugs (Fig. 2F). Figure 2. Metformin inhibits IDH1 expression and increases cancer cell sensitivity to chemotherapy 3.É‘-KG increases the sensitivity of metformin to chemotherapy É‘-KG is one of the products of the IDH1 enzyme. In this study, the authors treated endometrial cancer cells with metformin and found that the expression of É‘-KG was inversely proportional to the reaction time and dose of metformin (Fig. 3 A, B). É‘-KG was highly expressed in a cell line overexpressing the IDH1 gene, and after metformin treatment, É‘-KG expression was inhibited, indicating that metformin inhibited É‘-KG expression by inhibiting IDH1 gene expression (Fig. 3C). And when used in combination with anticancer drugs, it can effectively inhibit cell proliferation (Fig. 3D). Figure 3. α-KG is involved in metformin regulation of chemosensitivity 4. Metformin promotes the efficacy of anticancer drugs by regulating the IDH1-α-KG signaling pathway through TET1 methylolation As a kind of α-KG-dependent oxygenase, TET1 can promote the conversion of 5-methylated cytosine to 5-hydroxymethylcytosine. IDH1 acts as a precursor of α-KG. The function of binding to TET is still unclear. . Therefore, after overexpression of IDH1, the authors detected an increase in the mRNA and protein content of TET1 (Fig. 4A, B), and the expression level of α-KG also increased (Fig. 4C). By immunofluorescence experiments, it was observed that IDH1 or TET1 gene can increase intracellular methylolation levels, while metformin treatment or knockdown treatment, methylolation level is inhibited (Fig. 4D), and the dose and response of TET1 and metformin The time is consistent (Figure 4E). The Dot blot assay (Fig. 4F) again confirmed that the level of methylolation of IDH1 overexpressing cells was inhibited by metformin. Through cell proliferation experiments, after over-expression of TET1, the proliferation of cancer cells increased slightly, but chemotherapy drugs had little effect on cell proliferation (Fig. 4G). Figure 4. TET1 is involved in the sensitivity of metformin to endometrial cancer cells 5. The decrease of Nrf2 methylolation level affects the chemosensitivity caused by metformin Nrf2 and IDH1 are consistent in the distribution and onset of endometrial cancer tissues (Fig. 5A, B), and Nrf2 can bind to TET1 (Fig. 5C). It is known that TET1 affects the level of methylolation (Fig. 4D). Can Nrf2 affect the level of methylolation? The authors confirmed by Dot blot experiments that the methylation of Nrf2 gene is inseparable from the regulation of TET gene (Fig. 5D). The expression of Nrf2, TET1 and other genes in metformin-treated cells decreased at the protein level (Fig. 5E), and the level of methylolation in the Nrf2 promoter region decreased (Fig. 5F, G), indicating the role of Nrf2 in methylolation. May be on the promoter. After overexpressing the keap1 gene (in contrast to the Nrf2 gene), the cell proliferation activity was higher than that of the control group (Fig. 5H), indicating that the Nrf2 gene inhibited cell proliferation. In conclusion, metformin improves the sensitivity to chemotherapeutic drugs by affecting the TET1 gene and reducing the DNA methylolation modification of the Nrf2 promoter region. Figure 5. Metformin regulates the sensitivity of cancer cells to chemotherapeutic drugs by reducing the methylolation of Nrf2 6. Nrf2 gene enhances IDH1 signaling pathway through forward feedback As can be seen from the above, metformin is capable of inhibiting the expression of Nrf2 and related genes. Therefore, the authors wanted to see the trends in related genes after affecting the expression of the Nrf2 gene, and found that the expression trends were consistent (Fig. 6A-D). Moreover, inhibition of the Nrf2 gene promoted É‘-KG expression (Fig. 6E, F), that is, the Nrf2 gene regulates the IDH1-É‘-KG-TET1-Nrf2 signaling pathway. Subsequently, the authors predicted the structure of the IDH1 promoter region and found that there were six antioxidant response elements (ARE) in this region and the NF2 binding site (Fig. 6G), and found that one of the reporter genes was significantly associated with Nrf2. The above results indicate that Nrf2 regulates the IDH1-É‘-KG-TET1-Nrf2 signaling pathway by affecting IDH1 transcription. Figure 6. Nrf2 gene enhances IDH1 signaling pathway through forward feedback summary: This article is full of dry goods, not only reveals that metformin participates in the IDH1-É‘-KG-TET1-Nrf2 pathway, thereby inhibiting the development of endometrial cancer, and also confirms that TET1 and Nrf2 affect the level of DNA methylolation in the future. Endometrial cancer provides a theoretical basis for drug resistance to chemotherapeutic drugs. Full text information: Bai M, Yang L, Liao H, et al. Metformin sensitizes endometrial cancer cells to chemotherapy through IDH1-induced Nrf2 expression via an epigenetic mechanism. Oncogene(2018). Specially recommended products: Cloud-sequence combines methylolated DNA immunoprecipitation technology with high-throughput sequencing to provide a comprehensive DNA methylation sequencing service to help customers quickly access genome-wide DNA methylation profiles and different samples. Differential expression profile of the methylolation profile. With powerful E-Letter analysis capabilities, we provide you with massive sequencing data and publishing-level analysis images. Cloud order related product recommendation: DNA methylation sequencing DNA methylation sequencing m6A RNA methylation sequencing m5C RNA methylation sequencing m1A RNA methylation sequencing RIP sequencing RNA Pull Down Whole transcriptome sequencing Shanghai Yunxu Biological Technology Co., Ltd. Shanghai Cloud-seq Biotech Co.,Ltd Address: 3rd Floor, Building 20, No. 518, Zhangzhu Road, Songjiang District, Shanghai phone Website: mailbox: Tumor markers are substances, often proteins, that are produced by the cancer tissue itself or sometimes by the body in response to cancer growth. Because some of these substances can be detected in body samples such as blood, urine, and tissue, these markers may be used, along with other tests and procedures, to help detect and diagnose some types of cancer, predict and monitor a person`s response to certain treatments, and detect recurrence.
More recently, the idea of what constitutes a tumor marker has broadened. Newer types of tests have been developed that look for changes in genetic material (DNA, RNA), rather than proteins, in patient samples. The genetic changes have been found to be associated with certain cancers and can be used as tumor markers to help determine prognosis, guide targeted treatment, and/or detect cancers early on. Moreover, advances in technology have led to tests that can evaluate several genetic markers or panels of markers at the same time, providing expanded information about characteristics of a tumor.
Tumor Markers Tests,Prostate Specific Antigen Test,Alpha Fetoprotein Test,Carcinoma Embryonic Antigen Test,Fecal Occult Blood Test Changchun ZYF science and technology CO.,LTD , https://www.zyf-medical.com