What is the need to know when lung cancer treatment opens the "immune era"? October 27, 2016 Source: Pineapple Factor
Android system is an intercom system based on IP system.
It connects Video Door Phone and Indoor Monitor through POE Switch to achieve stable intercom and monitoring. It adopts TCP/IP networking architecture, and support multi-way unlocking and elevator linkage. Our intercom systems are developed by ourselves, which supports connection to mobile phone tuya without any indoor monitors, and it will be more convenient for customers who don`t want to use the indoor monitors, they can directly connect the video door phone through the tuya application and realize the remote intercom, monitoring and unlocking functions. So it can save more indoor monitor costs for customers. Sounds incredible, right? Then let me know more specifications and functions together!
Our Android intercom system uses a high-quality high-definition screen and a hard aluminum housing with 2pcs HD cameras design. It is full touch screen , so customers can operate like our mobile phones, which is very simple and convenient. At the same time it compatibles with and face recognition .RFID swipe card.tuya application and so on to open the door . Usually used for renovation of old communities, apartments and hotels, etc.
The following are the parameters of our AZZJ8A for your reference:
1. Operating system : Android 5.1.1
2. Screen Display : 8 inch screen
3. Camera : 200W(1080P)
4. CPU : ARM Cortex-A53SMP4
5. Memory : LPDDR3 1GB
6. Storage : EMMC 8GB (standard) expandable
7. Color : Silver/Champagne
8. Card Type : IC Card,M1,S50,13.56MHZ
9. Operation : Capacitive full touch screen
10. Communication : RJ45-TCP/IP
11. Power consumption : static less than 7W; Dynamic is less than 12W.
12. Power supply : DC18V-3A
13. Working environment : -20℃~70℃
14. Working humidity : 10%~70%
15. Protection grade : IP33
16. Unlock ways : RFID Card/Password/Face recognition/Mobile phone remote
17. Face recognition : Recognition distance ≥1M
18. Storage capacity 50,000
19. Recognition rate 0.5 seconds
20. Recognition rate. 99.98%
21. TUYA Function :snapshot/intercom/video record/ doorbell door unlock/record/monitoring/channel switching
22. Customization: language.ad photo.Logo and UI display
Our factory is located in Zhuhai, China. We have been focusing on independent research and development of intercom systems for 11 years. We can provide you with professional products and services, as well as complete intercom solutions according to your requirements. If you want to know more, please contact us and we can send a catalogue for your reference.
FAQ
Q: What is your service ?
a. we can offer OEM&ODM services
b. All software free provide , and technical support after-service
c. Online installation guide services.
Q: Whats the MOQ?
To ensure the best logistic cost, we suggest 100pcs(one carton).However, we are open to take orders with small qty.Welcome to sample order, contact with us.
Q: What payment terms are available?
T/T, Paypal, Western Union, credit card.
Q: How long is your production lead time?
Sample Order-Within 1-5 working days.
Bulk Order - 15-20 days for neutral items, 7 days if the items we have stock
Q: What's Your Products Warranty?
We Offer 12 Months Warranty after shipment sent.
Android Intercom System,Android Video Intercom,Wireless Intercom System,Video Intercom System Zhuhai Mingke Electronics Technology Co., Ltd , https://www.mingke-tech.com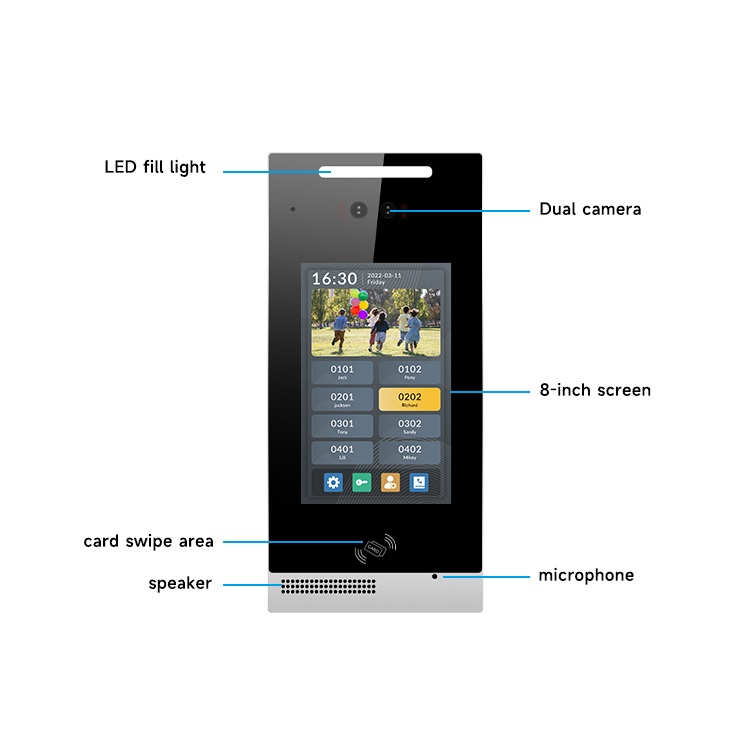
What is the need to know when lung cancer treatment opens the "immune era"?
The "immune era" does not mean that all lung cancer patients will use immunotherapy, but rather immunotherapy, which has become one of the mainstream choices parallel to chemotherapy and targeted drugs.
Â
It is important to note that Keytruda does not apply to all lung cancers at present, and is currently only for "non-small cell lung cancer" with "high expression of PD-L1" and "no EGFR or ALK mutation".
Â
This pile of technical terms may make everyone very happy. What is first-line treatment? Why is the PD-L1 protein highly expressed? How do you know if it is high expression? Can EGFR or ALK mutant lung cancer be used? and many more.
Â
Pineapple is here to talk to you about things that are not clearly stated in the news.
Â
(two)
Â
First, Keytruda is not the first, not the only immune drug used to treat lung cancer.
Â
On the time approved for lung cancer, the first award belongs to another well-known PD1 inhibitor Opdivo. It was first marketed in March 2015 for lung squamous cell carcinoma with second-line chemotherapy failure. In October, its application extended to second-line treatment of other non-small cell lung cancers, including adenocarcinoma.
Â
Keytruda is the second eye, and in October 2015 it was approved for second-line treatment of non-small cell lung cancer.
Â
Just last week, the FDA approved the third immunologic drug, the PD-L1 inhibitor Tecentriq, for second-line treatment of non-small cell lung cancer.
Â
Lung cancer immunotherapy is currently the three-legged Opdivo, Keytruda, and Tecentriq.
Â
But they are all approved as "second-line treatment," that is, only for patients who have failed first-line chemotherapy or targeted drugs.
Â
The reason why this news is "heavy", the core vocabulary is "first-line treatment."
Â
For newly diagnosed cancer patients, the first treatment is called “first-line treatmentâ€. If there is no effect, or after drug resistance, then it will receive “second-line treatmentâ€, and so on, and “three-line†and “four-line†treatment.
Â
Becoming a first-line treatment, it is the highest reward for any new drug. This first proves that the drug is currently the best choice, and more importantly, it will be used by more patients, bringing greater economic returns to the company.
Â
Although Keytruda is not the first immunization drug to treat lung cancer, it has overtaken the curve and became the world's first first-line immunotherapy for lung cancer.
Â
This is significant for patients because it means that many lung cancer patients will no longer need chemotherapy first, but can use immunization drugs directly.
Â
With the addition of immunotherapy, first-line treatment for non-small cell lung cancer will become more accurate:
Â
There are mainstream mutations such as EGFR and ALK, and priority is given to using targeted drugs.
Â
There is no mainstream mutation, but PD-L1 expression is high, considering the priority use of immunological drugs.
Â
There are no major mutations, and PD-L1 expression is low, considering preferential use of chemotherapy, or combination therapy.
Â
With the same disease, we are a little closer to "precise medicine."
Â
(three)
Â
Keytruda has become the first-line drug for this type of patient because it has broken the current first-line drug in clinical trials: chemotherapy.
Â
In the overall survival curve below, blue represents patients using Keytruda (also known as pembrolizumab), and survival rates are significantly higher than chemotherapy.
Â
Look at a few other important data:
Â
The proportion of objective response (significant tumor shrinkage), 44.8% vs 27.8%, immune therapy is better!
Â
Median progression-free survival, 10.3 vs 6.0 (months), immunotherapy won!
Â
The proportion of serious side effects, 26.6% vs 53.3%, immune therapy is better!
Â
Chemotherapy has been a first-line treatment for lung cancer for many years. Although effective, it has been limited by the low response rate (20%-30%) and the side effects. Now there is a sudden emergence of immunotherapy, from the efficacy to the side effects of comprehensive rolling chemotherapy.
Â
Just like suddenly, Tiangong had a Sun Wukong, and there was a Zhou Botong. There was a Mao Zedong in the East.
Â
In short, it’s all about changing the rules of the game.
Â
Immunization has entered the front line of lung cancer treatment and will change many rules. This is why everyone is so excited.
Â
Keytruda should be just the beginning. For many lung cancer patients without EGFR, ALK mutations, a variety of immunotherapy will gradually replace chemotherapy, becoming the mainstay of first-line treatment.
Â
But chemotherapy will not disappear, it will slowly retreat to second-line therapy. Similar to the current EGFR mutant lung cancer patients, usually the first line, even the second line are first targeted drugs, and then resistant to chemotherapy.
Â
(four)
Â
As I said earlier, not all lung cancer patients are suitable for using Keytruda as a first-line drug. There are three important conditions for this approval:
Â
"Non-small cell lung cancer"
Â
"No EGFR or ALK mutations"
Â
"Tumor cells are more than 50% positive for PD-L1"
Â
There are so many restrictions, there are clinical trial design reasons, and there are biological reasons.
Â
Why limit "non-small cell lung cancer"?
Â
This is because this clinical trial only recruits such patients to participate, not that immunotherapy is not effective for "small cell lung cancer." In fact, the data released this year show that immunotherapy is effective for 10%-20% chemotherapy failure of "small cell lung cancer", and several phase 3 clinical trials are currently underway, which is worth looking forward to.
Â
Why do you require "no EGFR or ALK mutations"?
Â
On the one hand, it is because the patients with these two mutations have a good effect on the use of targeted drugs in the first line. Therefore, when the clinical trials are recruited, mainly patients who do not have these mutations are recruited, and they need new therapies more;
Â
On the other hand, there are currently clinical data showing that patients with EGFR and ALK mutations have far less overall response rates to immunologic drugs than patients without mutations, and the scientific principles are still under investigation.
Â
Why limit "tumor cell PD-L1 positive more than 50%"?
Â
Because Keytruda is most likely to work for such a tumor.
Â
why?
Â
Cancer cells can grow and must find ways to escape immune system surveillance. Different tumors use different strategies, some of which rely on high expression of PD-L1 protein. The PD-L1 protein on the cell binds to the PD1 protein on the immune cells, and once they hold hands, they produce a signal that inhibits the activity of the immune cells.
Â
As a PD1 inhibitor, Keytruda's task is to break up PD1 and PD-L1 and destroy the harmonious relationship between cancer cells and immune cells. If the tumor is PD-L1 positive, it is likely to rely on the PD1/PD-L1 system to evade immune system attacks, so Keytruda has the highest probability of onset. Conversely, if the tumor expresses little PD-L1, it may be mainly by other means to escape the immune system regulation. Then using Keytruda for them, the effect is probably not good.
Â
Because of this, Keytruda was limited to "lung cancer cells with more than 50% positive PD-L1".
Â
For example, PD-L1 is like Princess Jianing. It is a cancer cell that has a good relationship with immune cells. It is specially sent to the door to "marriage" with PD1 (Wu Yingxiong) to ensure their safety.
Â
Keytruda, Wei Xiaobao, manages how many princesses you have, and seduce them all. This will irritate the immune cells and kill the cancer cells.
Â
"The tumor cell PD-L1 is more than 50% positive" is equivalent to saying "a large number of princesses are found here." At this time, Wei Xiaobao was given a great chance to take away the princess and destroy the "marriage."
Â
However, if "there is no princess found here", it indicates that cancer cells are likely to be surrounded by immune cells in other ways, and Wei Xiaobao is much less likely to be useful.
Â
What should I do? Then we have to analyze the specific issues.
Â
If it is Huang Rong, put Guo Jing, it is Ziwei, put it on Kang Kang, it is 甄嬛, put the fruit king. In short, our goal is to achieve "precise seduce."
Â
(Fives)
Â
Finally, simply answer a few readers' concerns, and then have the opportunity to expand specifically:
Â
1: How do you know if PD-L1 expression is more than 50%?
Â
Specific staining of tumor samples. Keytruda uses the "22C3 PharmDx" kit approved by the FDA last year to specifically detect PD-L1 expression. There are some other dyeing methods on the market, including China, which are similar.
Â
2: The expression level of PD-L1 is low. Is it illegal to use immunotherapy?
Â
No. The current consensus is that PD-L1 expression is high, and the overall effect of PD1 or PDL1 immunization drugs is better. But specific to the individual, it is difficult to predict. Patients who do not express PD-L1 respond to immunotherapy (but are indeed at a lower rate, about 5-10%). Conversely, patients with high PD-L1 expression are not effective with immunotherapy. In conclusion, the use of PD-L1 expression alone to predict the effectiveness of immunotherapy can increase the probability, but not absolute.
Â
This is a bit like saying "Sichuan people love to eat spicy, Cantonese people do not eat spicy", overall it is correct, but there are also Sichuan people do not eat spicy, but also Cantonese people love to eat spicy. If a person is found to be very spicy, the probability of being a Sichuanese is greatly increased, but not absolute.
Â
Because immunotherapy is very expensive, it is imperative to find ways to better predict the effects of immunotherapy.
Â
3: Chemotherapy, even targeted drugs, will gradually be replaced by immunotherapy?
Â
will not. Immunotherapy is a supplement to, rather than a substitute for, chemotherapy and targeted drugs. Different patients are treated with different therapies.
Â
The future direction is not to eliminate chemotherapy or targeted therapy, but to study how best to combine these therapies with immunotherapy to achieve optimal disease control. For example, due to the "immune lead effect" of chemotherapy, the use of "low-dose chemotherapy" to assist immunotherapy in the future can not only avoid the side effects of high-dose chemotherapy, but also probably better than immunotherapy alone. Pineapple believes this is the direction of development.
Â
4: Can EGFR and ALK mutant lung cancer patients be treated with immunotherapy?
Â
Targeted drugs are still recommended for first-line treatment in patients with EGFR and ALK mutations. But how to choose after the final drug resistance is the use of immune drugs or chemotherapy, there is no final conclusion.
Â
Chemotherapy is now the standard of choice, but just last week, the data for the drug Tecentriq unexpectedly showed efficacy in patients with EGFR or ALK mutations and drug-resistant drugs, and was therefore approved for second-line treatment in these patients. . Pineapple personally feels that this has yet to be confirmed by more data after the market launch. If this is the case, it will undoubtedly give patients a choice that is less than the side effects of chemotherapy.
Â
5: Will there be better treatments than Keytruda?
Â
I hope there is, and I firmly believe that there is! From the experimental data, even after screening, Keytruda still only works for less than 50% of patients, and there is still room for improvement. We must continue to work hard.
Â
The future of cancer treatment must be the world of combination therapy.
Â
The immunotherapy represented by Keytruda, Opdivo, and Tecentriq will be the cornerstone of many cancer treatments, but if you want to control the tumor for a long time, most patients need to add X. This X may be another immunization drug, or it may be radiotherapy, chemotherapy, or targeted drugs. For example, the combination of immunotherapy with PD1+CTLA4 is much better in melanoma than PD1 or CTLA4 alone. Their clinical trials in lung cancer are ongoing and can be better than Keytruda and will be known in the next year or two.
Â
What combination is best can only be proved by clinical trials.
Â
The good news is that now everyone is in full swing and only for lung cancer, there are now nearly 200 clinical trials in progress.
Â
Look forward to more breakthroughs together.