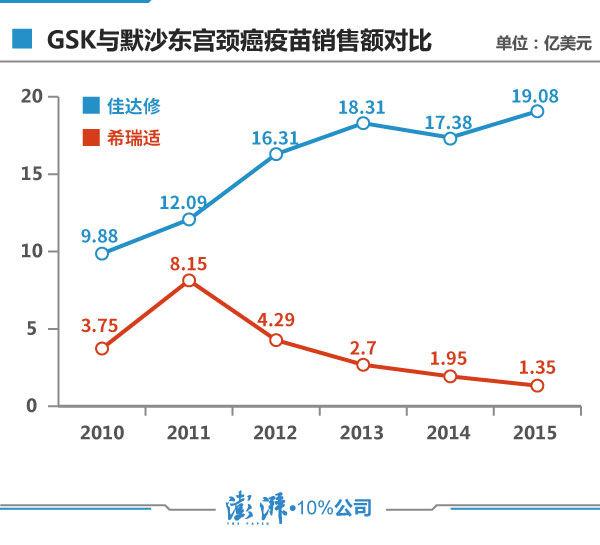
Cervical cancer vaccine---Mershadong's "Jia Daxiu" has always been ahead of GlaxoSmithKline's "Hirsch". October 27, 2016 Source: Phoenix Information According to a foreign medical professional website Fierce Pharma reported on October 21, a spokesman for GlaxoSmithKline revealed to him: "Because of the US market for human papillomavirus (HPV) vaccine (commonly known as cervical cancer vaccine) "Cervarix" (Cervarix) The demand is very low, and GlaxoSmithKline has decided to delist it from the US market." On October 25th, the relevant person in charge of GlaxoSmithKline China confirmed the news to the news (). The person in charge said that the delisting was due to insufficient demand of the company in the US market. Foreign media reported that GlaxoSmithKline spokesperson pointed out: "Because the United States has similar products on the market, the market demand for Shirei is very low, we based on this consideration to stop supplying to the US market, but Shirley is FDA The US Food and Drug Administration's marketing approval is still valid, and the company has been approved by 107 regulatory agencies, covering 136 countries around the world." According to the data, in 2015, the sales volume of “Cirrus†was 135 million US dollars, of which the US market was about 5 million US dollars, and the share was less than 4%. In the first half of this year, the global sales of “Cirrus†was 48 million. In the US dollar, the US market sold a total of 1 million US dollars, and its share has dropped to about 2%. This year coincides with the 10th anniversary of the launch of GlaxoSmithKline's "Hirsch" vaccine. In fact, the United States is also one of the earliest markets for the "Hirsch". In 2006, the US pharmaceutical company Merck was the first to develop the human papillomavirus (HPV) vaccine "Gardasil" and launched it in the US market. Soon after, GlaxoSmithKline immediately launched the world's second cervical cancer vaccine, "Cervarix." In these 10 years, Merck and GlaxoSmithKline have separated the major markets for cervical cancer vaccines worldwide. Both have been direct competitors in this field. However, from the perspective of sales in the global market, Merck's “Jia Da Xiu†has always been ahead of GlaxoSmithKline's “Hirschâ€. At first, the gap between the two sales was not too big, but as time went by, the sales of Jiada Xiu steadily increased, and the market share of “Xi Rui Shi†was shrinking all the way. According to the data, the annual sales volume of “Jia Da Xiu†in 2010 was 2.6 times that of “Xi Rui Shiâ€, and by 2015 the gap between the two sides has expanded to 14 times. Some analysts believe that the widening gap between the two may be related to the introduction of a more advanced vaccine by Merck. "Now the bivalent cervical cancer vaccine is outdated, which may explain why GlaxoSmithKline is delisting from the US." Li Ting, a senior researcher at Gao Tejia Medical Investment Group, told the news. According to the data, the "Jia Daxiu" that was introduced in 2006 is a four-valent vaccine. In 2014, Merck also launched a nine-price upgrade product, "Jia Da Xiu 9", and GlaxoSmithKline has been selling two-price "hi" “Rui Shi†– “price†represents the type of virus subdivision covered by the vaccine. The higher the price, the larger the coverage. It is understood that "Xi Rui Shi" can prevent 16 and 18 HPV viruses, and cervical cancer caused by these two viruses accounts for 70% of the total. The four-valent “Jia Da Xiu†can be used to prevent four HPV virus subtypes of 6, 11, 16, and 18, while “Jia Da Xiu 9†adds 31, 33, and 45 to the original four-price. The five HPV virus subtypes of 52 and 58 also reached 90% coverage of the virus. However, the relevant person in charge of GlaxoSmithKline denied this statement to the news, saying that the decline in demand in the US market is affected by the US public health policy and has nothing to do with the virus coverage of the product. In July of this year, GlaxoSmithKline’s “Xi Rui Shi†was approved in China after 10 years of long review, and became the first human papillomavirus vaccine listed in mainland China (the nine-valent vaccine currently listed in Hong Kong) ). “Xi Rui Shi†listed in mainland China is still a divalent product. According to previously announced news, the specific time to market for the vaccine is early 2017. On October 25, the reporter confirmed to GlaxoSmithKline that the relevant person in charge said that the “Xi Rui Shi†listing plan has not changed, but the specific time has not been determined. At the same time, the news reporter also learned from Merck East China that Merck is still actively promoting the listing approval of the four-valent HPV vaccine in China. Merck said to the news that the company is also preparing for the clinical trial of the nine-valent vaccine in China while planning the marketing of the four-valent vaccine. In addition to GlaxoSmithKline and Merck, there are also domestic companies that are stepping up their clinical trials of HPV vaccines, including Xiamen Wantai Bohai Biotechnology Co., Ltd. and Shanghai Zerun Biotechnology Co., Ltd., a subsidiary of Watson Bio (300142). Wantai disclosed to the media in March 2015 that the company's HPV vaccine is nearing completion of Phase III clinical phase and will be available within two years. Personal Protection Prouduct,Personal Protection Products,Personal Protection Equipment,Personal Protection Prouduct Equipment joho(HK) Technology Co. , Ltd. , https://www.joho-safety.com