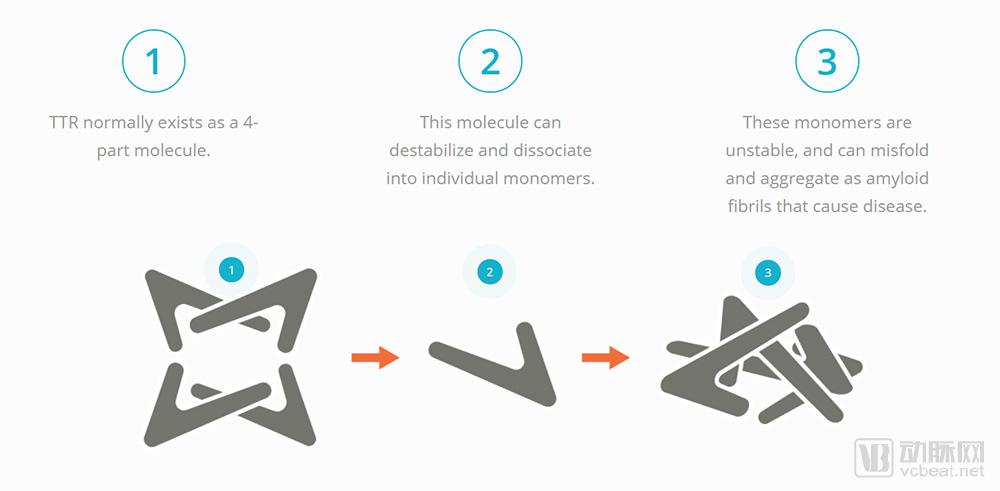
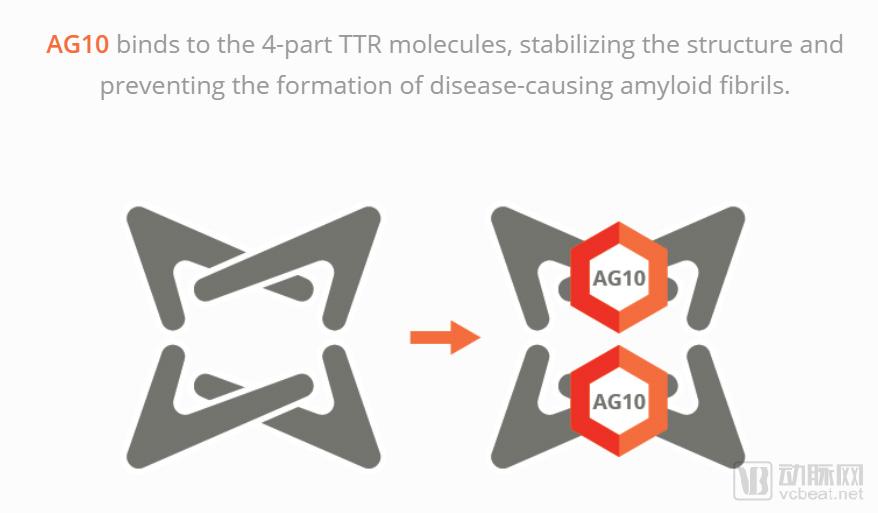
Due to the low incidence of rare diseases, the research resources they can obtain are very limited. Some pharmaceutical companies are also interested in the development, manufacture or introduction of drugs and foods for rare diseases based on economic interests, plus many diseases. There are not many cases, and it is very difficult to study these diseases. Even if a symptomatic drug is developed, because the patient market is relatively small, its price is also surprisingly high, and it is not affordable for patients. BridgeBio Pharma, a biopharmaceutical company from Palo Alto, USA, announced the formation of a new subsidiary, Eidos Therapeutics (Eidos), which will fund a $27 million investment in the newly formed company. Founded in 2013, Eidos is a clinical-stage biopharmaceutical company led by industry veterans focused on addressing amyloidosis (ATTR) caused by transthyretin (TTR). This is a rare disease in which medical needs have not yet been met, and the company is trying to treat such diseases by stabilizing TTR. What is TTR and ATTR? The main function of TTR is to maintain normal levels of thyroxine and retinol binding protein (RBP) in humans, and transport about 15% of thyroxine under normal physiological conditions. The content of TTR can reflect the changes of protein synthesis and content in the body after injury. In clinical practice, TTR plays an important role in the detection, prognosis and treatment of various diseases. ATTR is an amyloid caused by a variety of reasons, and their deposition in various organs of the body leads to the development of multiple neurological diseases and cardiomyopathy. Amyloidosis is a large amount of soluble fiber, soluble protein (amyloid) tissue that damages normal tissue function, leading to gradual depletion of affected organ function. Each year, approximately 250,000 people worldwide suffer from heart and neurological diseases due to TTR amyloidosis, and the risk of illness increases as the population ages. By stabilizing TTR and preventing the formation of toxic amyloid fibrils, Eidos developed the drug candidate AG10 to fight disease and gradually conduct clinical trials. According to the arterial network, AG10 developed by Eidos is a small molecule for oral administration, which aims to effectively stabilize the tetrameric TTR, thereby blocking a series of molecular reactions that produce ATTR from the source. The binding of AG10 to the tetrameric TTR can generate strong molecular bonds at the T119M mutation site, making TTR "super stable" and enhancing patient survival. Eidos CEO Neil Kumar purchased 1,000,000 shares of EIDX at an average price of $17 per share on June 22, 2014. The total cost of this purchase was $17 million. In April 2018, Eidos completed a $64 million Series B round of financing. The round of financing was led by RA Capital Management and included Eidos parent company BridgeBio Pharma and a number of new investors Janus Henderson, Viking Global Investors, Aisling Capital, Perceptive Advisors, Cormorant Asset Management and Amzak Health Investors. Eidos said the proceeds from the financing will be used to push Eidos' small molecule candidate AG10 into Phase 2 clinical trials and prepare for Phase 3 clinical trials. Recently, the company has filed an application with the US Securities and Exchange Commission, and an initial public offering (IPO) raised $115 million. Accurate drug learning and research and development become valuable experience "Our clinical data indicate that AG10 has good safety and tolerability, is capable of stabilizing 100% plasma TTR at peak concentrations, and provides an average stable level of >95% (plasma TTR) at steady state," Dr. Neil Kumar, CEO of Eidos, said. “Given the increasing level of stability that has gradually improved clinical results in past trials, our near-stable levels indicate that AG10 may be the industry's leading solution. We stabilize the TTR to target the source of ATTR. Verification of genetics and clinical data." Since March 2016, Dr. Neil Kumar has served as CEO and board member of Eidos. In September 2014, Dr. Kumar founded BridgeBio Pharma as its CEO, focusing on the learning and development of genetic diseases and precision medicine. The company provides drugs for cardiology, neurology, oncology, dermatology and endocrine diseases. BridgeBio Pharma was founded in 2015 and is headquartered in Palo Alto, California. From 2012 to 2014, Dr. Kumar served as the temporary vice president of the business development segment at MyoKardia. MyoKardia, a biotechnology company based in South San Francisco, USA, is committed to exploring a new molecular strategy to attack heart disease. Their small but potentially groundbreaking heart disease research has obtained the company's expected proof-of-concept efficacy data. In August 2017, the share price of MyoKardia soared by 81%. In addition, from 2007 to 2012, Dr. Kumar had many years of leadership experience at Third Rock Ventures and McKinsey & Company. McKinsey & Company is a world-leading global management consulting firm. Since its founding in 1926, the company has helped leading organizations achieve significant, lasting business performance improvements and create outstanding organizations that attract, nurture and motivate outstanding people. They have more than 80 branches in 44 countries around the world and have more than 7,000 consultants. Dr. Kumar holds a bachelor's and master's degree in chemical engineering from Stanford University and a Ph.D. in chemical engineering from the Massachusetts Institute of Technology. Since October 2016, Dr. Jonathan Fox has served as President and Chief Medical Officer (CMO) of Eidos. At the same time, Dr. Fox is responsible for the treatment and guidance of cardiovascular and renal diseases at BridgeBio Pharma. From March 2013 to March 2017, Dr. Fox served as CMO and Senior Consultant at MyoKardia, where he has extensive practical experience in medical consulting and corporate management. From 1993 to 2013, Dr. Fox taught at the University of Pennsylvania School of Medicine and is currently a part-time teacher at the Cardiovascular Institute at Stanford University. In addition, he received a bachelor's degree in biology, a master's degree in medicine and pathology from the University of Chicago, and completed training in internal medicine and cardiology at Duke University. Dr. Fox received the American College of Internal Medicine (ABIM) Cardiovascular Disease Certificate and is a principal investigator of the American College of Cardiology. How does AG10 solve the TTR amyloidosis problem? “TTR amyloidosis is a progressive, fatal disease, and there are no FDA-approved therapies to treat this disease. Currently, patients and their families can only rely on some supportive therapies, but this cannot be fundamentally cured. The disease does not change the course of the disease," said Jonathan Fox, president of Eidos. "Eidos is committed to developing a safe and effective drug to help patients solve TTR amyloidosis problems. AG10 has the potential to help companies accomplish this. A mission." TTR usually consists of four molecular moieties that can be broken down into four monomolecular structures. These unstable single molecules undergo an abnormality after folding, and then aggregate to form amyloid fibrils, which eventually cause disease. Eidos' scientific co-founder uses Stanford Medical's TRAM and SPARK programs to discover drugs using the latest medical technology to develop AG10. AG10 binds and stabilizes TTR in the blood in a small molecule mode, preventing the formation of amyloid, and maximally preventing further progression of the disease. The treatment candidate was co-directed by Eidos' experienced scientists and clinicians and entered the first phase of clinical trials in 2017. Eidos' submission to the US Securities and Exchange Commission shows: "In the Phase 1 clinical trial, after taking the highest dose of AG10 in healthy volunteers, we observed an overall TTR stability of more than 95% on average, when the AG10 plasma concentration peaked. The stability can reach 100%. In contrast, in our preclinical studies, 20 mg and 80 mg of tauamidis reached only 45% and 60% stability at peak levels, respectively. We believe these observations are due to the advantages of the AG10 binding pattern and specific binding to TTR, but not to other plasma proteins." In May 2018, Eidos took AG10 in the first phase of an ATTR cardiomyopathy patient. The company plans to include 45 patients with symptomatic ATTR cardiomyopathy in a randomized, double-blind, placebo-controlled trial. At least 30% of patients in the trial developed mutant ATTR cardiomyopathy, and the remaining patients were wild-type. Patients will receive a placebo or two different doses of AG10 at a random rate of 1:1. If well tolerated, the company expects to maintain the treatment plan for 28 days. Comparison of Eidos Therapeutics and competitors 1. Comparison of Eidos and Avrobio Avrobio, one of the first companies to treat rare diseases, is one of the first companies to enter the clinical phase, focusing on gene therapy for lysosomal storage diseases. Patients are mainly lacking enzyme or protein genes that provide important metabolic functions, including Fabry diseases, Gaucher disease, cystinosis, and Pompe disease. The company applies its proprietary lentiviral gene therapy to lysosomal storage diseases, as well as other widespread diseases that use gene therapy to achieve therapeutic benefits. The lentiviral vector technology steadily adds genes to the patient's own cells, integrating the patient's permanent genome to provide a lifelong, lasting cure. Avrobio designed the advanced third-generation 4-plasmid lentiviral vector platform, a highly efficient, proven gene transfer system that stably adds genes to patients' own CD34+ stem cells. Avrobio's platform includes patented technology, unique manufacturing processes, know-how and tools for gene therapy. Compared to Eidos, Avrobio helps patients with rare diseases recover normal genes through gene transfer, cell extraction, isolation, and lentiviral gene injection therapy, the principle of which is very different from Eidos' molecular therapy. Avrobio received $60 million in Series B financing in February 2018 and plans to list on NASDAQ in the US, hoping to receive $86.25 million in IPO fundraising. 2. Comparison of Eidos and Arrowhead Pharmaceuticals Arrowhead Pharmaceuticals is a biopharmaceutical company headquartered in Pasadena, Calif., with a full-time employment of 104 people, mainly engaged in the development of new drugs for intractable diseases. Arrowhead's Targeted RNAi (TRiMTM) molecular platform utilizes ligand-mediated delivery for the purpose of enabling simple, tissue-specific targeting for innovative pharmaceuticals. Arrowhead's clinical phase products include ARC-520, an RNAi-based therapy for chronic hepatitis B virus infection; and ARC-AAT, a novel unlocked nucleobase analog containing RNAi-based therapeutics for treatment and Alpha-1 antitrypsin lacks related diseases. The two drugs are currently in clinical trials at the llb and lb phases, respectively. Compared with Eidos, Arrowhead has been established for a long time, with rich clinical research results and nearly $22.2 million more than Eidos. Organic Siberian Ginseng Extract
Organic Siberian ginseng extract powder is extracted from the roots, stems, and leaves of Siberian ginseng. Siberian Ginseng or Eleuthero has been used in China for 2000 years as a folk remedy for bronchitis, heart ailments, and rheumatism, and as a tonic to restore vigor, improve general health, restore memory, promote healthy appetite, and increase stamina.
Referred to as [Ci Wu Jia" in Chinese medicine, it was used to prevent respiratory tract infections as well as colds and flu. It was also believed to provide energy and vitality. In Russia, eleuthero was originally used by people in the Taiga region of Siberia to increase performance and quality of life and to decrease infections.
Siberian Ginseng Extract,Siberian Ginseng Extract Powder,Organic Siberian Ginseng Extract Powder,Organic Siberian Ginseng Extract Organicway (xi'an) Food Ingredients Inc. , https://www.organicwayinc.com