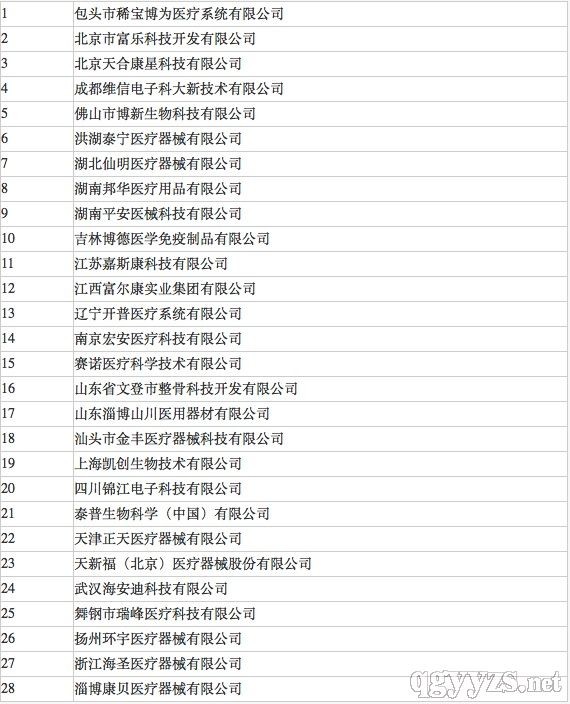
From June 13th to 17th, the second training course for medical device inspectors, hosted by the CFDA Medical Device Supervision Department and hosted by the Senior Institute of Advanced Education, was successfully held in Beijing. A total of 60 people from the medical device supervision departments and business backbones of 31 provinces across the country participated in the training. Trainees with qualified training and examination will participate in flight inspection and overseas inspection in the later stage, and will be awarded the second batch of national inspector certificates in due course. The second batch of state-level inspectors: high-level, strict assessment It is reported that this training course is based on the national medical device inspector training plan, based on the summary of the two-month advanced training course on quality management of medical device production. The second inspector advanced training course. Sun Lei, deputy director of the Department of Medical Device Supervision of the General Administration of the State Council, attended the training class and delivered a speech. The course also invited a number of experts with extensive international inspection experience and senior inspectors from the system to teach. Trainees who have passed the training assessment will participate in flight inspection and overseas inspection in the later stage, and issue the second batch of national inspector certificates in due course. Participants in the training must pass the strict closed-loop examination and pass the qualifications to become the second batch of national inspectors for medical devices. CFDA's first batch of state-level medical device inspectors had a total of 35 people, and the second batch of 60 people participated in the training. The number of people who could finally stay and successfully recruited as national inspectors is still unknown. However, in accordance with the publicity of the first batch of national inspectors, the second batch of lists will also be made public. 50 domestic machinery enterprises will meet the flight inspection! There are also overseas inspections! What does a national inspector mean? According to the CFDA website announcement, 28 medical device manufacturers have accepted flight inspections from late March to late June this year, and several of them have been ordered to suspend production and rectification. The implementers of these flight inspections were the first batch of state-level medical device inspectors. In the three months, 28 inspections of machinery enterprises were conducted, and the density and intensity of inspections were far higher than in previous years. The first batch of national inspectors have demonstrated their power to the industry and should have not been checked. Household Supplies,Household Items,Household Goods,Household Mini Electric Appliances Ningbo Shuangtuo International Trade Co., Ltd. , https://www.nbst-sports.com