Detailed Product Description  Weight Loss Fat Loss Hormones Drug Rimonabant CAS 168273-06-1 for Reduceing Weight      Detailed Product Description  Weight Loss Fat Loss Hormones Drug Rimonabant CAS 168273-06-1 for Reduceing Weight    Â
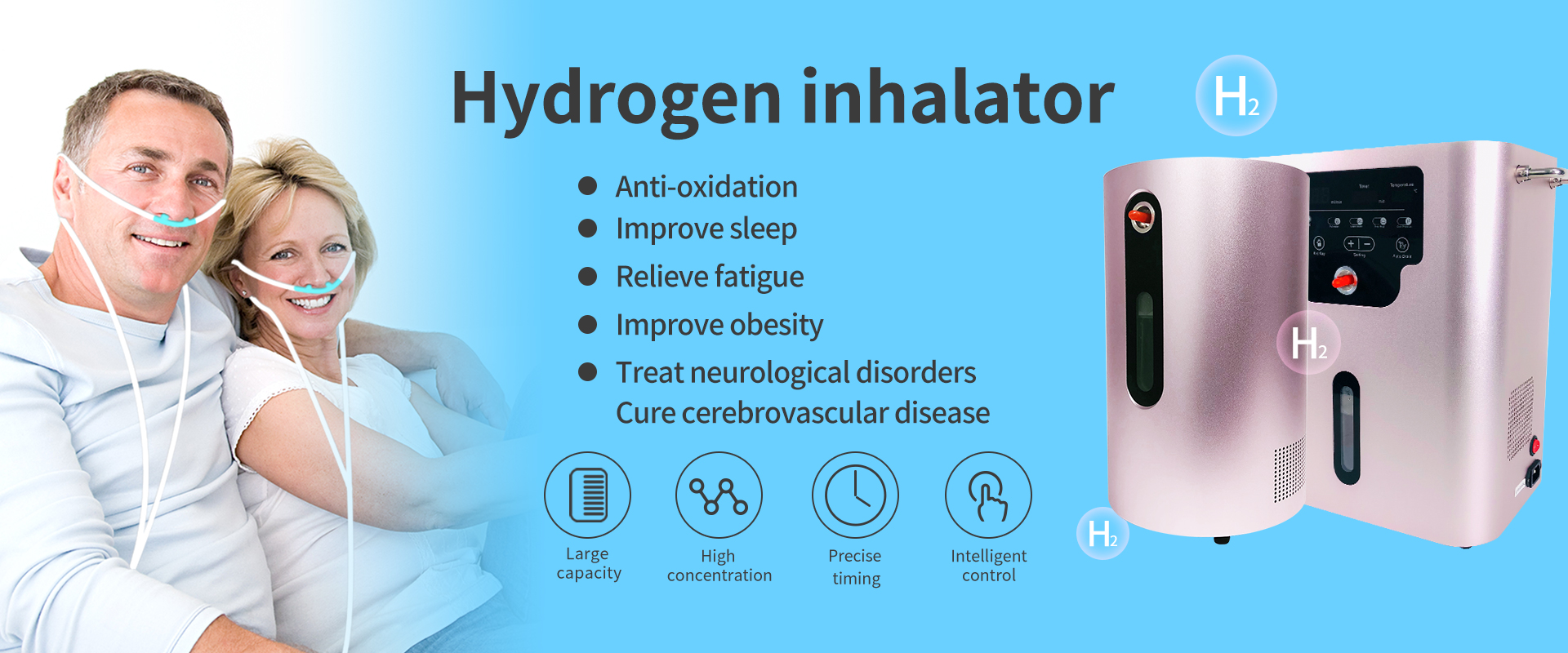
Hot Sale Portable Household Spe Pem Electrolysis Gas Hydrogen Breathing Inhalation Device Pure Water Electrolyzer Hydrogen Production Generator
Japanese Hydrogen Inhaler,Water Electrolysis Hydrogen Generator,Hydrogen Production Machine,Brown Gas Generator,Hydrogen Inhalation Machine
A hydrogen inhaler is a device that delivers hydrogen gas for inhalation. Hydrogen gas is considered to have potential health benefits due to its antioxidant and anti-inflammatory properties. The inhaler typically contains a chamber that generates hydrogen gas by electrolysis of water or by using hydrogen-rich tablets or cartridges. When the user inhales through the device, they can potentially benefit from the therapeutic effects of hydrogen gas. Some claimed benefits of hydrogen inhalation include reducing oxidative stress, inflammation, and improving athletic performance. However, further research is needed to fully understand the potential benefits and risks associated with hydrogen inhalation. It is important to consult a healthcare professional before using any new health device or treatment.
What Is Hydrogen?
1. Reduce oxidative damage
Japanese Hydrogen Inhaler,Water Electrolysis Hydrogen Generator,Hydrogen Production Machine,Brown Gas Generator,Hydrogen Inhalation Machine Shenzhen Guangyang Zhongkang Technology Co., Ltd. , https://www.nirlighttherapy.com
Name:
Rimonabant
CAS:
168273-06-1
Assay:
98%
Powder:
Yes
Sjipping Methods:
EMS,HKEMS,FEDEX,DHL,TNT,Aramex,ETC
Â
Quick Details
Product Name
Rimonabant
Synonyms
Acomplia;5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-piperidinopyrazole-3-carboxamide;1H-Pyrazole-3-carboxamide, 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-1-piperidinyl-;5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide
CAS
168273-06-1
EINECS
N/A
MF
C22H21Cl3N4O
MW
463.79
Standard
Enterprise Standard
Assay
98%
Chemical Properties
White to off white powder
Usage
Rimonabant is a selective antagonist of CB1 with IC50 of 13.6 nM and EC50 of 17.3 nM in hCB1 transfected HEK 293 membrane.
Packing
Foil bag
Rimonabant Description
Â
(1) Rimonabant hydrochloride (SR-141716A) is a potent and selective CB1 cannabinoid inverse agonist/antagonist with a Ki of 1.6 nM, minimal affinity for CB2, and and some GPR55 agonist activity.
Â
Rimonabant was developed as an anti-obesity drug because of its appetite suppressant activity, but was taken off the market because of side effects of depression and anxiety.
Â
(2) Under development by Sanofi-Aventis, Acomplia (rimonabant) is a selective CB1 endocannabinoid receptor antagonist indicated for the treatment of obesity.
Â
(3) It works by blocking endogenous cannabinoid binding to neuronal CB1 receptors. Activation of these receptors by endoegenous cannabinoids, such as anadamide, increases appetite. It is the most advanced endocannabinoid receptor antagonist in clinical development and offers a novel therapeutic approach to appetite control and weight reduction.
Â
Rimonabant Application
Â
Rimonabant is a weight loss drug, Sanofi-Aventis by the French company successfully developed for the treatment of obesity is the first cannabinoid type 1 receptor (CB1) antagonist, by selectively blocking the EC system cannabis Su -1 (cannabinoid-1, CB1) receptors play a role, can significantly reduce weight, reduce waist circumference, reducing risk factors for cardiovascular disease, but also can improve blood lipids and insulin resistance and metabolic syndrome, especially for the existence of clinical obesity or heart disease risk for people.
Â
Rimonabant may also be found to be effective in assisting some smokers to quit smoking.Sanofi is currently conducting studies to determine the possible value of rimonabant in smoking-cessation therapy. The Studies with Rimonabant and Tobacco Use (STRATUS) program involves more than 6,000 subjects.
Â
STRATUS is designed to explore two smoking-related therapies: first, to use rimonabant directly to aid in smoking cessation; second, to help prevent weight gain in former smokers. Initial results apparently suggest rimonabant is effective for both uses. However, the FDA has explicitly stated to Sanofi that, without additional studies, rimonabant cannot be approved in the United States for smoking cessation therapy.
COA
Test Items
Specification
Test Results
Description
White crystalline powder
White crystalline powder
Identification
IR , UV conform
Conform
Loss on drying
≤0.5ml
0.21ml
Residue on ignition
Not more than 0.2%
0.06ml
Iron
Not more than 0.005%
0.0018%
Heavy metals
Not more than 0.001%
0.0006%
Related substances
Total: Not more than 1.0%
Individual: Not more than 0.5%0.32%
0.14%
E-isomer
Not more than 0.3%
0.19%
Organic volatile impurities
Conforms
Conforms
Assay
99.0~101.0%
99.51%
Conclusion
Conform with USP30
What is Weight Loss Steroids Rimonabant (Acomplia) Used for?
Â
Acomplia was studied to be used complementary to diet and exercise to treat obese or overweight patients who suffer from type 2Â diabetes and abnormal levels of fat in the blood.
Â
Sanofi argued that Acomplia could also prevent the risk of cardiovascular disease. Patients with large waist circumference (102 cm in men and 88 cm in women) were said to most benefit from taking the drug.
Â
Rimonabant was submitted to the Food and Drug Administration (FDA) for approval in the United States. However, in 2007, the FDA's Endocrine and Metabolic Drugs Advisory Committee (EMDAC) concluded the French manufacturer Sanofi-Aventis failed to demonstrate the safety of rimonabant and voted against recommending the anti-obesity treatment for approval. Subsequently, Sanofi-Aventis announced it was suspending the new drug application (NDA) for rimonabant, and that it would resubmit an application at some point in the future.
Â
Rimonabant may also be found to be effective in assisting some smokers to quit smoking. Sanofi is currently conducting studies to determine the possible value of rimonabant in smoking-cessation therapy. The Studies with Rimonabant and Tobacco Use (STRATUS) program involves more than 6,000 subjects. STRATUS is designed to explore two smoking-related therapies: first, to use rimonabant directly to aid in smoking cessation; second, to help prevent weight gain in former smokers. Initial results apparently suggest rimonabant is effective for both uses. However, the FDA has explicitly stated to Sanofi that, without additional studies, rimonabant cannot be approved in the United States for smoking cessation therapy. According to a Cochrane Collaboration review in 2007, rimonabant "may increase the odds of quitting approximately 11/2-fold".
Â
Related product
Â
Name
Molecular Formula
Formula Weight
CAS
Rimonabant
C22H21Cl3N4O
463.79
168273-06-1
Calcium pyruvate
C6H6CaO6
214.19
52009-14-0
Hydroxycitric acid
C6H8O8
208.12
6205-14-7
Kidney Bean Extract
N/A
N/A
85085-22-9
Orlistat
C29H53NO5
495.73
96829-58-2
Picolinic acid chromium(III) salt
C18H12CrN3O6
418.3
14639-25-9
Synephrine
C9H13NO2
167.21
34520
Theobromine
C7H8N4O2
180.16
83-67-0
5-Hydroxytryptophan
C11H12N203
220.22
56-69-9
Name:
Rimonabant
CAS:
168273-06-1
Assay:
98%
Powder:
Yes
Sjipping Methods:
EMS,HKEMS,FEDEX,DHL,TNT,Aramex,ETC
Â
Quick Details
Product Name
Rimonabant
Synonyms
Acomplia;5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-piperidinopyrazole-3-carboxamide;1H-Pyrazole-3-carboxamide, 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-1-piperidinyl-;5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide
CAS
168273-06-1
EINECS
N/A
MF
C22H21Cl3N4O
MW
463.79
Standard
Enterprise Standard
Assay
98%
Chemical Properties
White to off white powder
Usage
Rimonabant is a selective antagonist of CB1 with IC50 of 13.6 nM and EC50 of 17.3 nM in hCB1 transfected HEK 293 membrane.
Packing
Foil bag
Rimonabant Description
Â
(1) Rimonabant hydrochloride (SR-141716A) is a potent and selective CB1 cannabinoid inverse agonist/antagonist with a Ki of 1.6 nM, minimal affinity for CB2, and and some GPR55 agonist activity.
Â
Rimonabant was developed as an anti-obesity drug because of its appetite suppressant activity, but was taken off the market because of side effects of depression and anxiety.
Â
(2) Under development by Sanofi-Aventis, Acomplia (rimonabant) is a selective CB1 endocannabinoid receptor antagonist indicated for the treatment of obesity.
Â
(3) It works by blocking endogenous cannabinoid binding to neuronal CB1 receptors. Activation of these receptors by endoegenous cannabinoids, such as anadamide, increases appetite. It is the most advanced endocannabinoid receptor antagonist in clinical development and offers a novel therapeutic approach to appetite control and weight reduction.
Â
Rimonabant Application
Â
Rimonabant is a weight loss drug, Sanofi-Aventis by the French company successfully developed for the treatment of obesity is the first cannabinoid type 1 receptor (CB1) antagonist, by selectively blocking the EC system cannabis Su -1 (cannabinoid-1, CB1) receptors play a role, can significantly reduce weight, reduce waist circumference, reducing risk factors for cardiovascular disease, but also can improve blood lipids and insulin resistance and metabolic syndrome, especially for the existence of clinical obesity or heart disease risk for people.
Â
Rimonabant may also be found to be effective in assisting some smokers to quit smoking.Sanofi is currently conducting studies to determine the possible value of rimonabant in smoking-cessation therapy. The Studies with Rimonabant and Tobacco Use (STRATUS) program involves more than 6,000 subjects.
Â
STRATUS is designed to explore two smoking-related therapies: first, to use rimonabant directly to aid in smoking cessation; second, to help prevent weight gain in former smokers. Initial results apparently suggest rimonabant is effective for both uses. However, the FDA has explicitly stated to Sanofi that, without additional studies, rimonabant cannot be approved in the United States for smoking cessation therapy.
COA
Test Items
Specification
Test Results
Description
White crystalline powder
White crystalline powder
Identification
IR , UV conform
Conform
Loss on drying
≤0.5ml
0.21ml
Residue on ignition
Not more than 0.2%
0.06ml
Iron
Not more than 0.005%
0.0018%
Heavy metals
Not more than 0.001%
0.0006%
Related substances
Total: Not more than 1.0%
Individual: Not more than 0.5%0.32%
0.14%
E-isomer
Not more than 0.3%
0.19%
Organic volatile impurities
Conforms
Conforms
Assay
99.0~101.0%
99.51%
Conclusion
Conform with USP30
What is Weight Loss Steroids Rimonabant (Acomplia) Used for?
Â
Acomplia was studied to be used complementary to diet and exercise to treat obese or overweight patients who suffer from type 2Â diabetes and abnormal levels of fat in the blood.
Â
Sanofi argued that Acomplia could also prevent the risk of cardiovascular disease. Patients with large waist circumference (102 cm in men and 88 cm in women) were said to most benefit from taking the drug.
Â
Rimonabant was submitted to the Food and Drug Administration (FDA) for approval in the United States. However, in 2007, the FDA's Endocrine and Metabolic Drugs Advisory Committee (EMDAC) concluded the French manufacturer Sanofi-Aventis failed to demonstrate the safety of rimonabant and voted against recommending the anti-obesity treatment for approval. Subsequently, Sanofi-Aventis announced it was suspending the new drug application (NDA) for rimonabant, and that it would resubmit an application at some point in the future.
Â
Rimonabant may also be found to be effective in assisting some smokers to quit smoking. Sanofi is currently conducting studies to determine the possible value of rimonabant in smoking-cessation therapy. The Studies with Rimonabant and Tobacco Use (STRATUS) program involves more than 6,000 subjects. STRATUS is designed to explore two smoking-related therapies: first, to use rimonabant directly to aid in smoking cessation; second, to help prevent weight gain in former smokers. Initial results apparently suggest rimonabant is effective for both uses. However, the FDA has explicitly stated to Sanofi that, without additional studies, rimonabant cannot be approved in the United States for smoking cessation therapy. According to a Cochrane Collaboration review in 2007, rimonabant "may increase the odds of quitting approximately 11/2-fold".
Â
Related product
Â
Name
Molecular Formula
Formula Weight
CAS
Rimonabant
C22H21Cl3N4O
463.79
168273-06-1
Calcium pyruvate
C6H6CaO6
214.19
52009-14-0
Hydroxycitric acid
C6H8O8
208.12
6205-14-7
Kidney Bean Extract
N/A
N/A
85085-22-9
Orlistat
C29H53NO5
495.73
96829-58-2
Picolinic acid chromium(III) salt
C18H12CrN3O6
418.3
14639-25-9
Synephrine
C9H13NO2
167.21
34520
Theobromine
C7H8N4O2
180.16
83-67-0
5-Hydroxytryptophan
C11H12N203
220.22
56-69-9
1. Hydrogen is a chemical element, the first on the periodic table.
Is Hydrogen Safe?
Weight Loss Fat Loss Hormones Drug Rimonabant for Reduceing Weight
Model NO.: 168273-06-1
Trademark: Desen Nutrition
Transport Package: Box-Packed
Specification: 10g/foil bag
Origin: China
HS Code: 3001200010
Model NO.: 168273-06-1
Trademark: Desen Nutrition
Transport Package: Box-Packed
Specification: 10g/foil bag
Origin: China
HS Code: 3001200010
2. The usual elemental form of hydrogen is hydrogen gas.
3. It is a colorless, odorless, highly flammable gas composed of diatomic molecules, lightest gas.
4. The smallest, lightest, most, most harmless and most obvious antioxidant effect in the world.
Hydrogen has fast movement speed and strong penetration. After entering the human body, it is very easy to reach all parts of the body and the inside of tissues and cells. For example, hydrogen can reach the mitochondria and other fine structures in the cells, fundamentally adjusting the state of cells.
1. The diver breathes hydrogen for diving
2. Official EU/US document - hydrogen is a food additive
3. Japan considers hydrogen inhalation a medical procedure
2. Anti-inflammatory
3. Reduce apoptosis
4. Remove malignant free radicals
5. Enhance immune function