Dragon fruit powder is rich in protein vitamins and trace elements such as selenium, calcium, phosphorus, potassium, iron, magnesium and natural pigments such as anthocyanins. Pitaya Powder,Red Pigment,Pitaya Fruit Powder,Dragon Fruit Powder Shaanxi Zhongyi Kangjian Biotechnology Co.,Ltd , https://www.zhongyibiology.com
It is well known that exercise can reduce the incidence of cardiovascular disease. The current research reports focus on muscle factors because of its paracrine and endocrine effects, which can coordinate the multi-system effects of exercise. However, the underlying mechanisms of exercise for cardioprotection are currently unknown. The latest research results of the research team of the Fourth Military Medical University and Wang Lifeng show that long-term exercise-derived circulating exosomes can transmit cardiac protection signals and identify exosome miR-342-5p as a new cardioprotective motility factor. It is able to counteract damage caused by myocardial ischemia and reperfusion (MI/R) in rats. 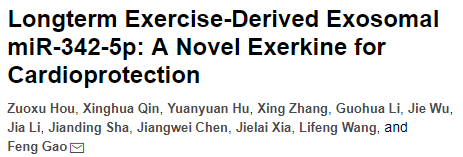
Impact factor: 15.211
Experimental method: miRNA sequencing
Experimental materials: plasma of humans and rats in long-term exercise (experimental group), plasma of non-sports and rats (control group) 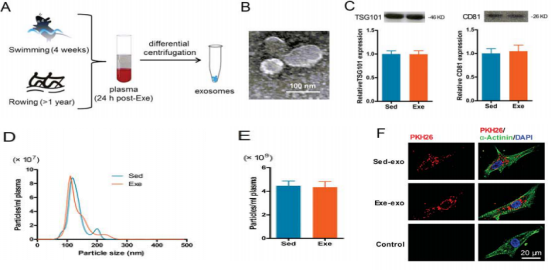
First, the authors performed myocardial ischemia for 30 minutes and reperfusion for 24 hours. It was found that compared with the sedentary control group (sed), the myocardial infarct size and LDH decreased in the exercise group (exe). Exercise has a significant myocardial protective effect on MI/R injury. 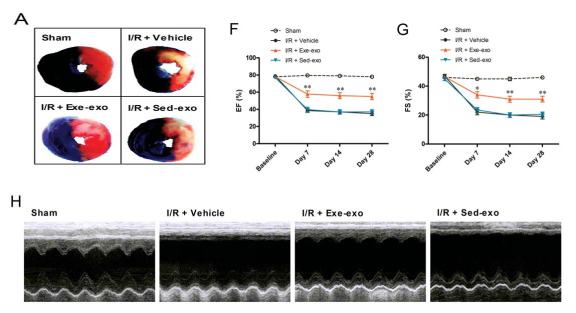
Figure 3. Exe-exo reduces myocardial infarct size and improves cardiac function caused by MI/R injury
To explore the possibility of miRNAs acting in exosomes-induced cardioprotection, the authors performed high-throughput sequencing of miRNAs in exosomes of two groups of rats (cloud order can provide this service) . A total of 14 differentially expressed miRNAs were detected (Fig. 4A). After further validation by q-PCR analysis, 12 differentially expressed miRNAs were identified, 11 of which were up-regulated (Fig. 4B). The authors then used hypoxia/reoxygenation (H/R) models to study how these 11 up-regulated miRNAs affect cardiomyocyte function. It was found that miR-342-5p can significantly reduce the apoptosis of cardiomyocytes under H/R, reduce the release of LDH, and increase cell viability (Fig. 4C-E). 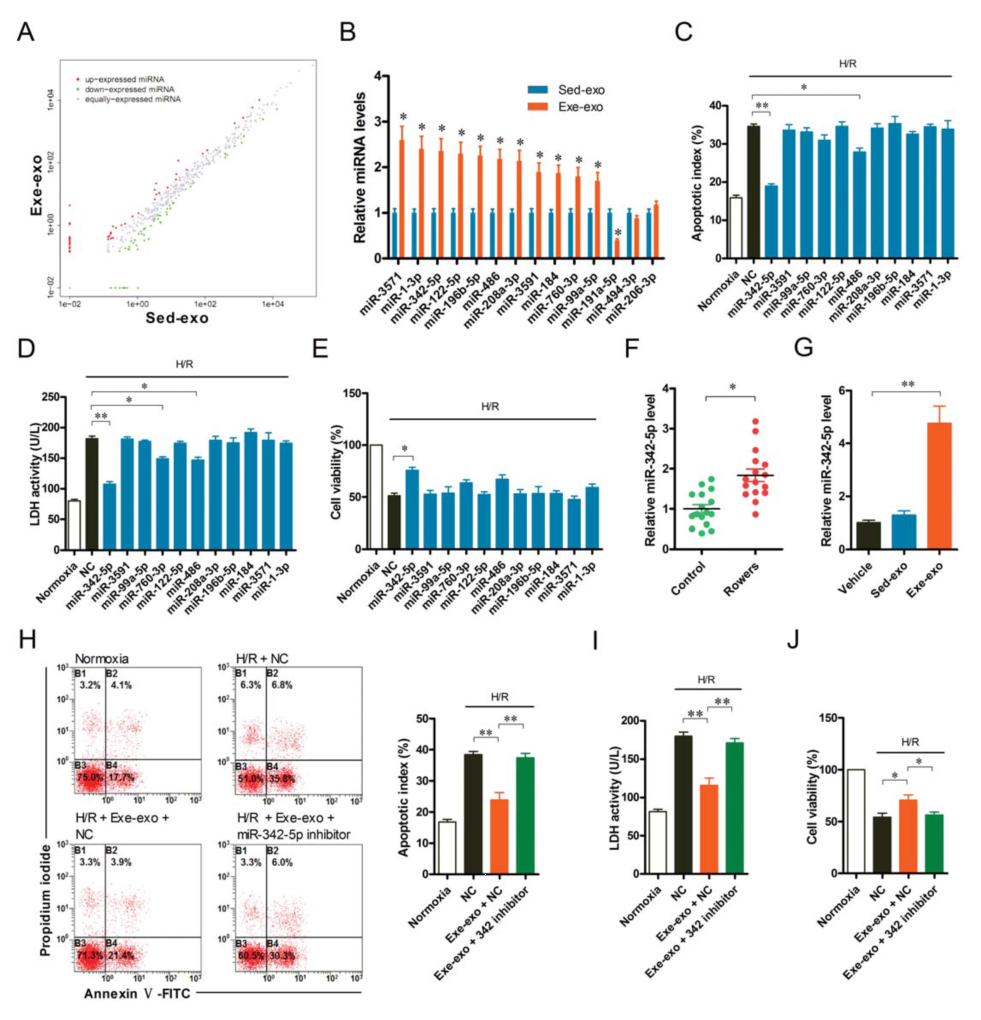
To further investigate the mechanism of action of miR-342-5p, the authors predicted the target gene of miR-342-5p and found that Caspase3, Caspase9 and Jnk2 are involved in the MI/R injury and apoptotic pathway (Fig. 5A). The authors also found that increased miR-342-5p significantly reduced the protein levels of Caspase9 and Jnk2, and vice versa, but no significant changes in Caspase3 gene expression were observed (Fig. 5B). The authors then further investigated the binding sites of the two genes Caspase9 and Jnk2 to miR-342-5p using luciferase (Fig. 5C). In addition, exe-exo incubation significantly inhibited H/R-induced cardiomyocyte lysis-Caspase3, cleavage-Caspase9 and phospho(P)-Jnk2 expression (Fig. 5D). Taken together, these data indicate that miR-342-5p exerts an anti-apoptotic effect in cardiomyocytes by targeting Caspase9 and Jnk2. 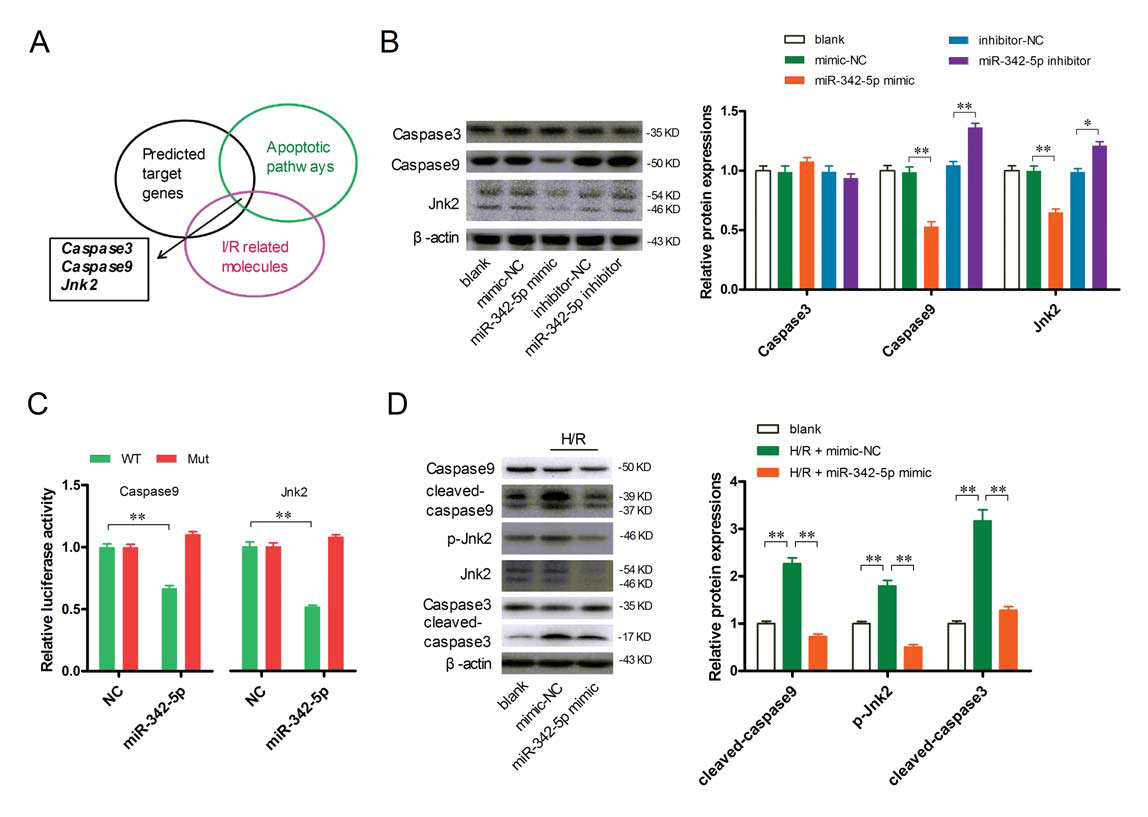
Survival signals AKT and ERK 1/2 have been identified as key molecules for cardioprotection. miR-342-5p up-regulated AKT phosphorylation in cardiomyocytes, whereas phosphorylation of ERK 1/2 did not change significantly (Fig. 6A); a similar phenomenon was observed in H/R cardiomyocytes (Fig. 6B-D) . These results indicate that miR-342-5p activates the survival signal AKT of cardiomyocytes. Interestingly, the authors found that Ppm1f is a potential target for miR-342-5p, the only phosphatase predicted by biosignal analysis (Fig. 6E). As shown in Figure 6F, miR-342-5p can reduce Ppm1f protein levels and vice versa. Luciferase reporter assay further confirmed that miR-342-5p can directly bind to the 3'UTR region of Ppm1f (Fig. 6G). Notably, down-regulation of Ppm1f protein expression not only significantly increased AKT phosphorylation (Fig. 6H-I), but also decreased H/R-induced cardiomyocyte apoptosis (Fig. 6J), suggesting that AKT phosphorylation may be elevated. Play a role in myocardial protection. 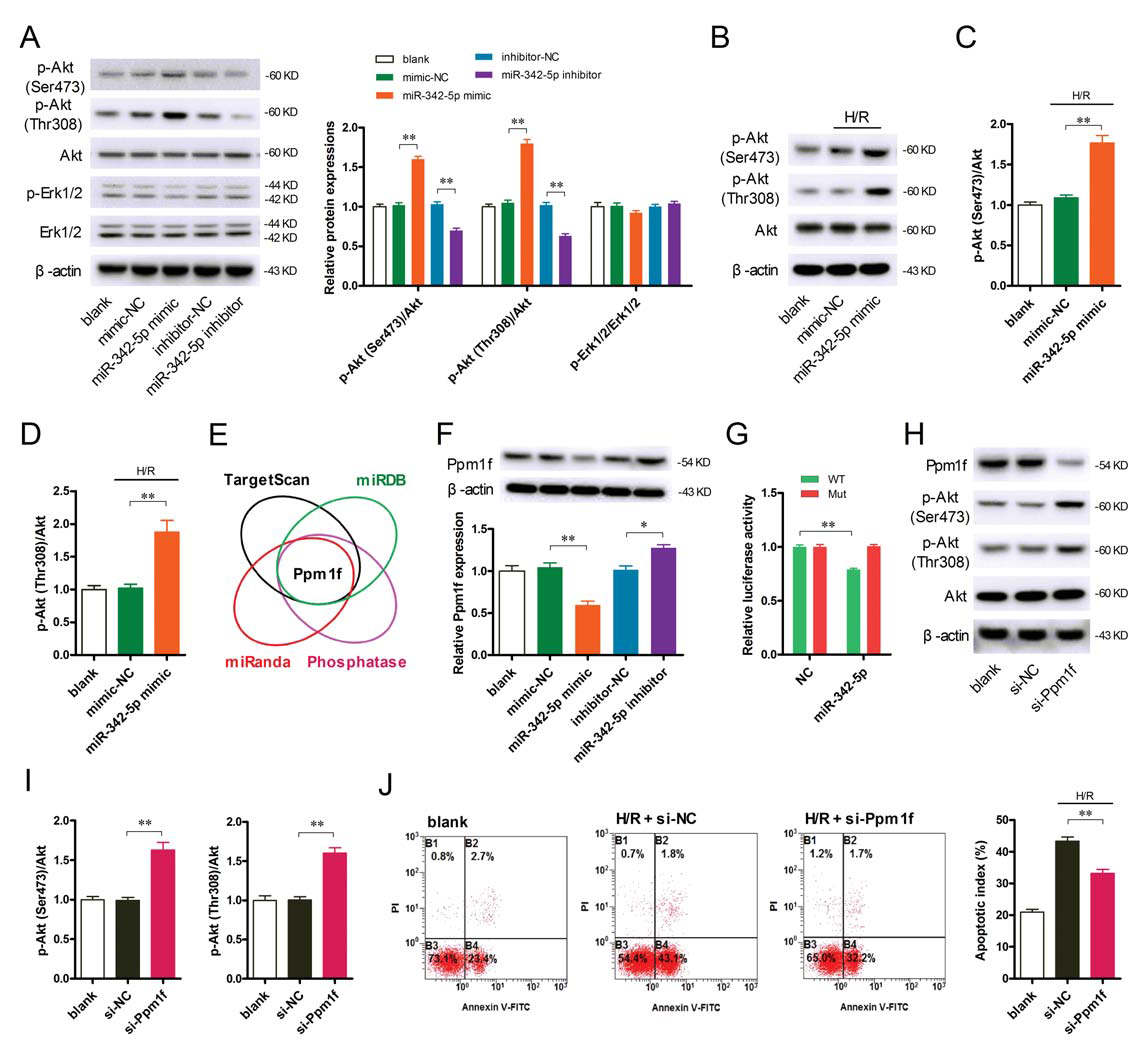
To investigate the long-term exercise-induced increase in circulating extracorporeal miR-342-5p in rats, the authors examined mature miR-342-5p in rat heart, skeletal muscle, aorta, liver, adipose tissue, kidney, spleen and plasma. Expression of its precursors revealed that mature miR-342-5p was expressed in multiple tissues (Fig. 7A). After exercise, the amount of mature miR-342-5p and precursor miR-342-5p in these tissues increased significantly. Unexpectedly, their increase mainly occurred in the aorta rather than skeletal muscle or cardiomyocytes. (Fig. 7B-C). Further studies showed that the amount of mature miR-342-5p and precursor miR-342-5p was significantly reduced after removal of aortic endothelial cells, suggesting that endothelial cells are the major source of aortic miR-342-5p (Fig. 7D). Next, the authors isolated aortic endothelial cells from exercised and sedentary rats and found that long-term exercise significantly increased mature miR-342-5p and precursor miR-342-5p in endothelial cells (Fig. 7E). In addition, electron microscopy revealed the presence of typical polycystic (MVBS) in the cytoplasm of the endothelium, where MVBS fuses with the plasma membrane and releases exosomes into the circulation (Fig. 7F). These results suggest that vascular endothelial cells are an important source of miR-342-5p in exosomes caused by exercise.
Blood flow-induced LSS is a motor-related physiological stimulator that is thought to be a central signaling mechanism for exercise-induced endothelial cell adaptation. To investigate the effect of LSS on miR-342-5p, human umbilical vein endothelial cells were cultured in resting conditions and LSS conditions. It was found that LSS not only increased the mature miR-342-5p and pre-miR-342-5p in the cells (Fig. 7K), but also increased the secretion of exosome miR-342-5p (Fig. 7L). This suggests that exercise-induced LSS can directly stimulate endothelial cell production and release of miR-342-5p. 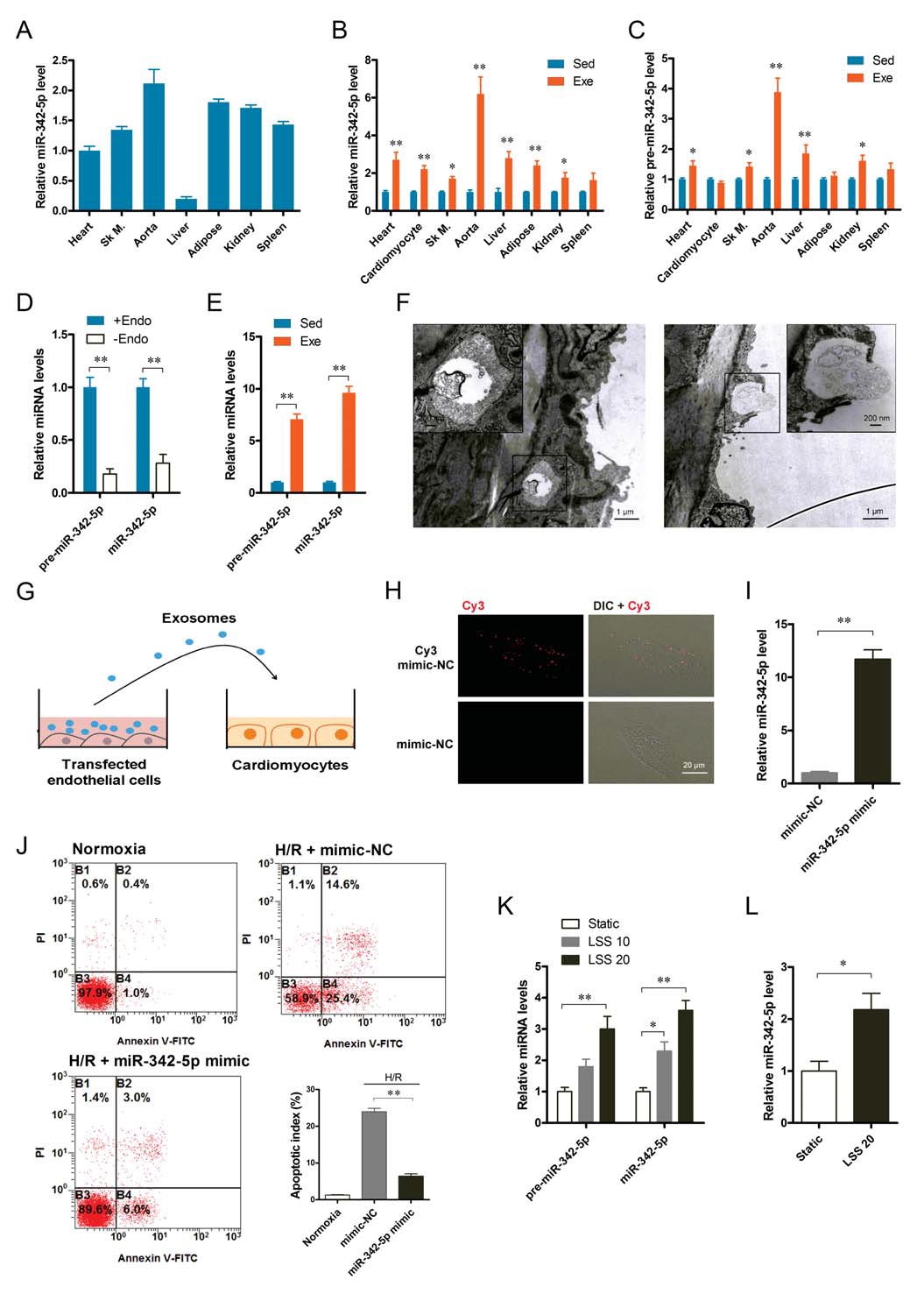
miR-342-5p can be absorbed by cardiomyocytes and protect cardiomyocytes from H/R damage;
LSS can directly stimulate endothelial cell production and release of miR-342-5p
To investigate the role of miR-342-5p in exercise-induced cardioprotection in vivo, the authors used a virus with a specific sequence (AAV9) to inhibit miR-342-5p in rat hearts. One week before the swimming training, AAV-anti-miR-342-5p or AAV negative control (Fig. 8A) was injected into the rat myocardium, and transfection of AAV9 was detected 24 hours after the training. As shown in Figure 8, exercise training increased miR-342-5p in the heart (Fig. 8B), while reducing Caspase 9, Jnk 2 and Ppm1f expression, and enhancing AKT phosphorylation; reducing miR-342-5p reduced these Change (Figure 8C). Importantly, after exercise, myocardial infarct size (Fig. 8D-E) and cardiomyocyte apoptosis (Fig. 8F-G) were significantly reduced in MI/R injury, and this was significant after reducing miR-342-5p. Weakened (Fig. 8D-G). Injection of miR-342-5p 48 hours prior to MI/R injury significantly increased miR-342-5p expression levels in the rat heart (Fig. 8H) and reduced myocardial infarct size (Fig. 8I). Taken together, these in vivo data suggest that miR-342-5p plays a crucial role in resistance to MI/R injury in long-term exercise-induced cardioprotection. 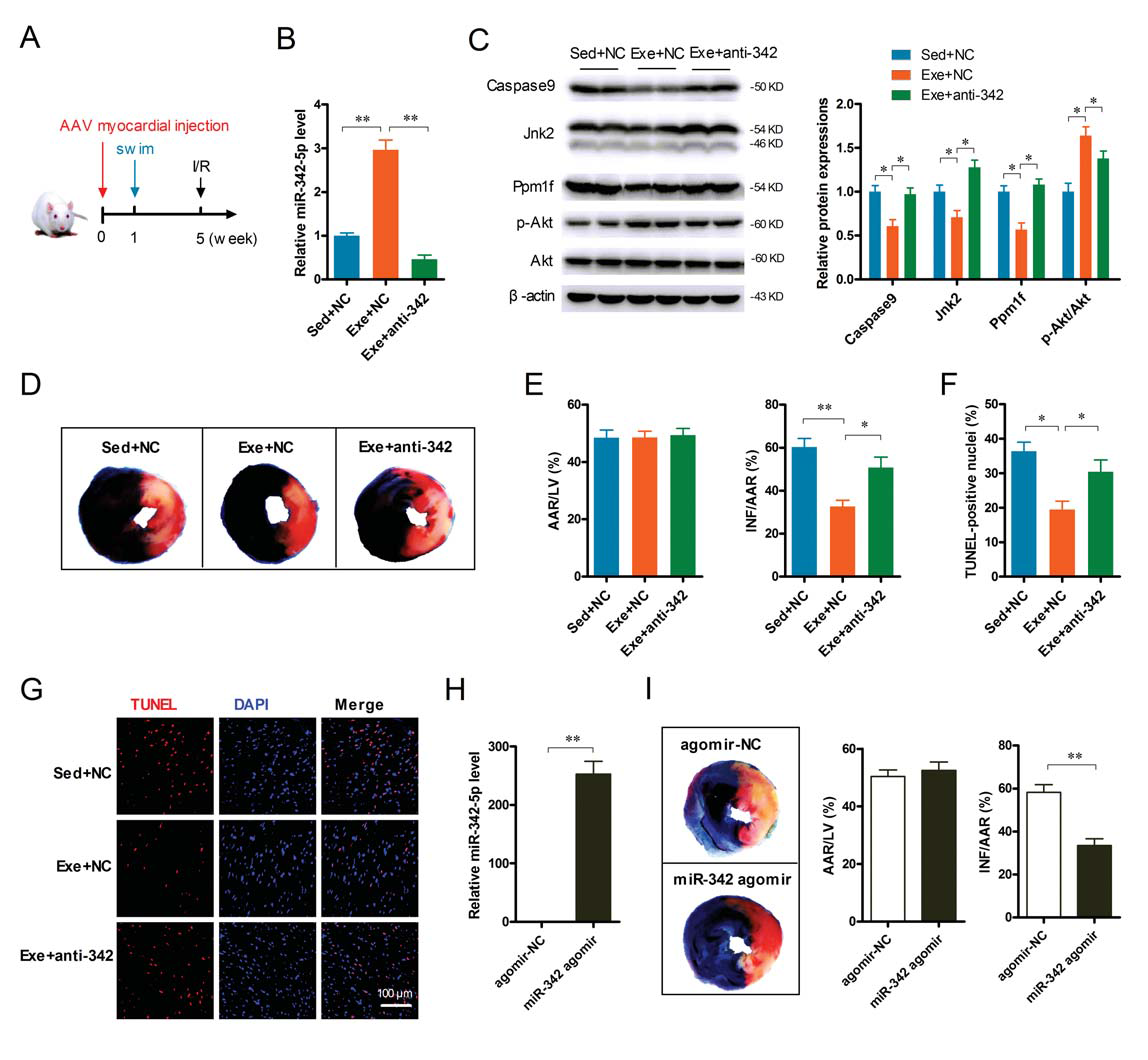
Long-term exercise-derived circulating exosomes, although their plasma levels did not change significantly, provided far-reaching and sustained cardioprotection for MI/R injury. The authors found that exosome miR-342-5p is a key cardioprotective molecule that inhibits apoptotic signals (Caspase9 and Jnk2) and enhances cardiac survival signals (P-Akt). Vascular endothelial cells may be the major source of exosomes miR-342-5p, indicating mutual interference between blood vessels and the heart in exercise-provided cardioprotection. These findings reveal that exercise-administered myocardial protection can serve as a novel mechanism of myocardial protection, highlighting the therapeutic potential of miR-342-5p in the prevention and rehabilitation of ischemic heart disease.
In addition to providing exosome miRNA sequencing, cloud-sequence organisms also cover the entire transcriptome of exosomes, circular RNA, LncRNA, and mRNA sequencing. More importantly, the cloud sequence not only solves the problem of exosome sequencing for the majority of scientific research users, but also provides a one-stop service for exosome extraction, identification and post-analysis. So far, 3000+ exosomes have been sequenced, helping many researchers to sprint high-scoring articles. Users only need to provide serum, plasma, cell supernatant and other samples, please contact us for details! 
https://
Cloud order related product recommendation:
Exosomes Whole Transcriptome Sequencing Exosomes miRNA Sequencing Exosomes Circular RNA Sequencing Exosomes LncRNA Sequencing Exosomes mRNA Sequencing
Past review:
Cloud-sequence organisms - exosomes one-stop service exosome identification cloud sequence organisms - exosomes transcriptomics of those things He Yunxu customers published the first brain trauma exosomes circular RNA article!
Cloud order customer 13 points top story, revealing that diabetic platelet-derived miRNA participates in sponge mechanism to mediate arterial injury repair
Shanghai Cloud-seq Biotech Co., Ltd.
Address: 3rd Floor, Building 20, No. 518, Zhangzhu Road, Songjiang District, Shanghai 
Telephone Fax Website:
mailbox:
The latest exosomes to study miRNA results: take you to reveal the benefits of long-term exercise on the heart
Article introduction
Published issue: Circulation Research
Article content
1. Exercise has no effect on the number of plasma exosomes
The authors of this article first collected the plasma of exercised and sedentary rats, and isolated exosomes. The exosomes in the plasma of exercise rats were experimental group (exe-exo), and the exosomes in the plasma of sedentary rats were the control group ( Sed-exo) (Fig. 1A), after exosome microscopy (Fig. 1B), the exosome proteins of both groups were tested. The results showed that there was no significant difference in the amount of exosomes between the two groups (Fig. 1C). And the NTA analysis also showed no significant difference in particle size distribution and plasma concentration between the two groups (Fig. 1D and E). The authors then co-cultured with pkh26-labeled exosomes and cardiomyocytes, and it was observed that exosomes were internalized by cardiomyocytes after 6 hours of incubation (Fig. 1F).
Figure 1. There is no difference in the number of exosomes in exercise and sedentary rats; exosomes can be internalized by cardiomyocytes
Then the question comes, does the above two conditions exist in the human body?
So the author did the same test on people. The plasma exosomes of the athletes were the control group (Row-exo) and the plasma exosomes of the common people were the control group (Con-exo), and the results were consistent with the rats (Fig. 2K and L). In addition, the authors also found that co-culture of athlete's exosomes with human cardiomyocytes reduced apoptosis (Fig. 2M), while releasing serum lactate dehydrogenase LDH (Fig. 2O) and increased myocardial cell viability (Fig. 2O) Figure 2N).
Figure 2. There is no difference in the number of exosomes between athletes and ordinary people; exosomes can also be internalized by cardiomyocytes.
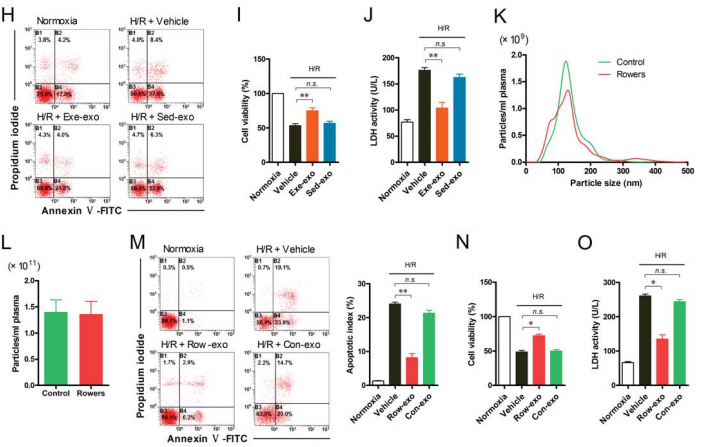
2. Exercise exocytosis (exe-exo) has myocardial protective effect on MI/R injury
To study the effects of exocrine secreted by exercise on myocardial protection, the authors isolated exosomes from equal volumes of plasma in both groups of rats and injected pkh26-labeled exosomes 48 hours before MI/R injury. Enter normal rat myocardium. The rats were then subjected to MI/R injury. After 24 hours, the infarct size of the rats injected with exe-exo was significantly reduced compared with the rats injected with sed-exo (Fig. 3A), accompanied by a decrease in LDH. In addition, the authors also found that the heart function of rats injected with exe-exo was improved, such as retention of ejection fraction and score shortened by 4 weeks after MI/R (Fig. 3F-H). In summary, exe-exo has a myocardial protective effect on MI/R injury.
3. miR-342-5p is a key component of exe-exo-induced heart protection
To better understand the function of exosome miR-342-5p, the authors examined the levels of plasma exosome-treated cardiomyocytes and miR-342-5p and its precursors in the heart. After exe-exo incubation and cardiac injection for 48 hours, the miR-342-5p level was significantly increased (Fig. 4G), while the precursor miR-342-5p level did not change significantly, indicating that exe-exo will miR -342-5p is delivered to cardiomyocytes or heart. Furthermore, inhibition of miR-342-5p significantly attenuated the protective effect of exercise-type plasma exosomes on the heart (Fig. 4H-J). These results indicate that miR-342-5p plays a key role in exosome-induced cardioprotection.
Does long-term exercise lead to differential expression of miR-342-5p in human exosomes?
The authors then examined the levels of miR-342-5p in plasma exosomes before and after athlete training, and found that its expression increased 1.8 times after training (Figure 4F).
Figure 4. miR-342-5p plays a key role in cardioprotection provided by exe-exo
4. miR-342-5p exerts anti-apoptotic effect by targeting Caspase 9 and Jnk2 in cardiomyocytes
Figure 5. miR-342-5p exerts anti-apoptotic effects by binding to Caspase 9 and Jnk2 in cardiomyocytes.
5.miR-342-5p enhances phosphorylation of AKT in cardiomyocytes by targeting Ppm1f
Figure 6. miR-342-5p targets Ppm1f to enhance phosphorylation of AKT in cardiomyocytes
6. Endothelial cells are the main source of miR-342-5p in sports secretory exosomes
Next, the authors investigated whether exosomes released by endothelial cells could be absorbed by cardiomyocytes. The authors used cy3 to label exosomes secreted by endothelial cells and added them to human cardiomyocytes (Fig. 7G). Confocal imaging confirmed that cy3 labeled miRNAs can be transported from endothelial cells to cardiomyocytes (Fig. 7H). Incubation of cy3-labeled exosomes with cardiomyocytes showed an increase in miR-342-5p in recipient cardiomyocytes (Fig. 7I). More importantly, receptor cardiomyocytes reduced apoptosis after H/R injury (Fig. 7J). Taken together, these results indicate that mi-342-5p released by endothelial cells can be absorbed not only by cardiomyocytes, but also by protecting cardiomyocytes from H/R damage.
7. Layer shear stress (LSS) stimulation can up-regulate the expression of miR-342-5p in endothelial cells
Figure 7. Endothelial cells are the major source of miR-342-5p in motor secretory exosomes;
8. miR-342-5p plays an indispensable role in long-term exercise-induced cardioprotection in vivo
Figure 8. miR-342-5p plays an important role in resistance to MI/R injury in exercise-induced cardioprotection
to sum up:
Cloud sequence bio-exosomes advantages summary:
Full text link:
Shanghai Yunxu Biological Technology Co., Ltd.