Fresh Fruit refers to the edible fruits of plants with juicy and mainly sweet and sour tastes. Fruit not only contains rich vitamin nutrition, and can promote digestion. AGOLYN offers a variety of fruits such as Fresh apples, Fuji apples, kiwi, pears, lemons and so on . Fresh Fruit,Fresh Apple,Kiwi Fruit, Fresh Lemon,Fresh Pears Xi'an Gawen Biotechnology Co., Ltd , https://www.seoagolyn.com
The general method is to collect analyte-related information (if known) and perform gradient or isocratic HILIC screening for three ACE HILIC phases (with three different ACE HILIC columns at different pH eluents) The experiment (depending on the hydrophilicity range of the sample analyte) is then optimized for chromatography to achieve an acceptable HILIC method.
The ACE HILIC screening conditions are listed in Table 1.
These designs are designed to explore a wide range of selectivity and provide a good starting point for achieving the desired separation.
Figure 17
ACE HILIC method development flow chart 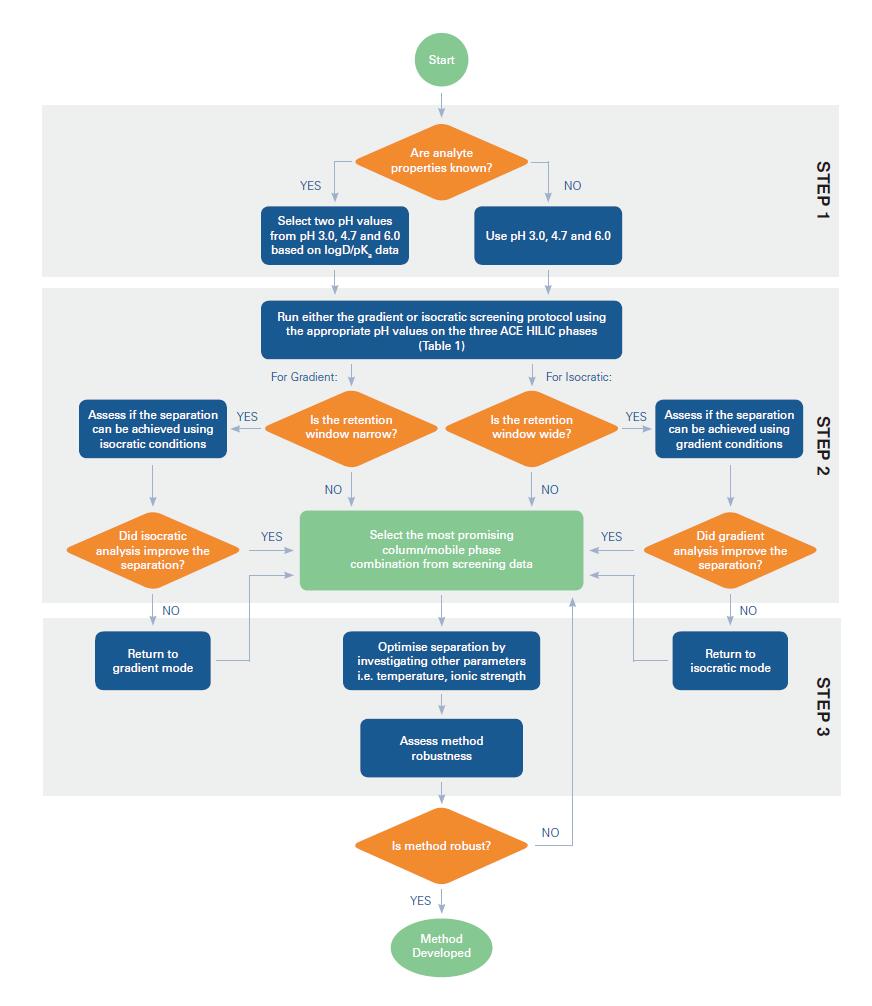
Table 1
ACE HILIC screening test conditions Parameter Remarks Column ACE HILIC-A, ACE HILIC-B and ACE HILIC-N, 150 x 4.6 mm, 5 μm Gradient mobile phase A: 10 mM ammonium formate (dissolved in MeCN/H2O) (94:6 v/v)
B: 10 mM ammonium formate (dissolved in MeCN/H2O) (50:50 v/v) ammonium formate pH: 3.0, 4.7 or 6.0.
Gradient screening Time %B 0 0 15 100 20 100 twenty one 0 41 0 Isocratic mobile phase 10 mM ammonium formate (dissolved in MeCN/H2O) (90:10 v/v) ammonium formate pH: 3.0, 4.7 or 6.0. Flow rate 1.5 mL/min temperature 25 °C Detection Depends on sample (depending on sample)
ACE HILIC Column Preservation Method <br> After use, the ACE HILIC column should be flushed with 7:3 by volume of acetonitrile and water to remove all buffer salts.
Then, rinse with 100% isopropanol at a lower flow rate for storage. Tighten the column end caps back (tighten the column end caps) and return the column to the box.
After each analysis run, unless the next day is to be used, it is recommended to clean the column in a closed method (by long-term storage) and then rinse with isopropanol.
Example 1 - Caffeine and Related Compounds <br> Caffeine and four related compounds (theobromine, theophylline, hypoxanthine and xanthine) required for method development are polar neutral substances, among which A negative log P value indicates reasonable hydrophilicity (log P is a negative value that clearly indicates its hydrophilicity) and is therefore suitable for HILIC (Figure 18).
Figure 18
Structure and log P data of caffeine and related substances 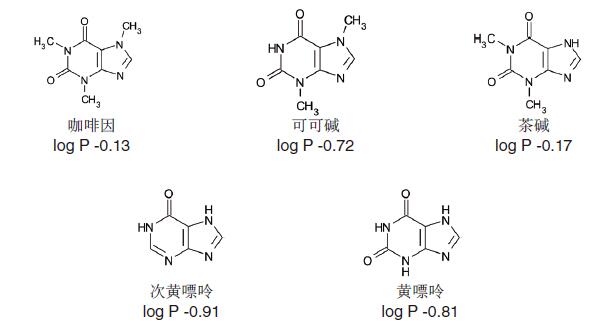
Caffeine and related compounds are not ionizable at pH 3-6, so the pH of the eluent hardly affects the molecule.
To do this, choose pH 3.0 and 4.7.
The stationary phase can be affected by changes in the pH of the eluent, which can be a beneficial side.
The ionization changes in the stationary phase will affect the hydration layer around the particles, which can affect the extent to which the analyte is dispersed into the phase (distributed on the stationary phase) or forms hydrogen bonds.
Therefore, the three stationary phases were screened at pH 3.0 and pH 4.7, and the results are shown in FIG.
The new ACE HILIC columns are equilibrated (balanced) with 60 column volumes to form a hydrated layer around the particles. The mobile phase, gradient and temperature are shown in Table 1.
The screening results showed that some selectivity differences were observed between the three stationary phases and the two pH values ​​(the screening results of the three stationary phases at two pH values ​​showed a difference in selectivity between each other).
The most efficient separation based on screening data was for the ACE HILIC-N phase at pH 3.0 (at pH 3.0).
These data selections (select these conditions) are used for further optimization.
Figure 19
Gradient screening of ACE HILIC columns 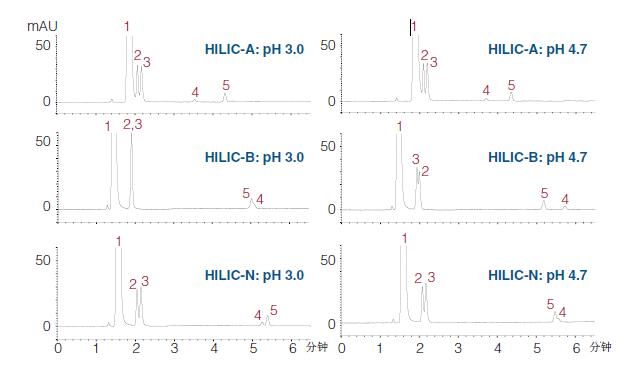
The conditions are as described in Table 1, except for the detection at 275 nm. Inject 2 μL of a 25 mg/mL caffeine mixture (using ammonium formate at pH 3.0 and pH 4.7, mixing the relevant substance and MeCN/H2O (90:10 v/v) in a ratio of 0.5% w/w)
sample:
1) Caffeine
2) Theophylline
3) Theobromine
4) Huang Wei
5) Hypoxanthine
The retention window (time period) of the earlier eluting peaks is very narrow, which means that the isocratic HILIC can be used to separate the analytes. (Isocratic HILIC can be used to isolate these compounds) 10 mM ammonium formate, pH 3.0 (dissolved in MeCN/H2O) (94:6 v/v) was selected as isocratic conditions (Figure 20).
Under these conditions, peaks 4 and 5 are retained more (good), but peaks 2 and 3 are still unresolved (separated).
According to the results of the gradient run, higher acetonitrile content does not appear to increase the resolution of peaks 2 and 3 (high acetonitrile content does not increase the resolution of the 2 and 3 peaks), so the gradient HILIC analysis is improved (so the gradient analysis is for the mixture) The separation is more suitable and the temperature will be studied) to develop the final method, as shown in Figure 21.
Figure 20
Isocratic analysis of caffeine and related compounds in ACE HILIC-N phase 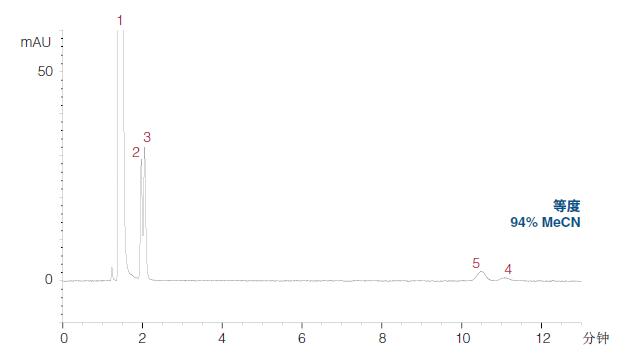
Isocratic analysis column for caffeine and related compounds in ACE HILIC-N phase: ACE 5 HILIC-N,
150 x 4.6 mm
Mobile phase: 10 mM ammonium formate, pH 3.0 (dissolved in MeCN/H2O) (94:6 v/v)
Flow rate: 1.5 mL/min
Temperature: 25 °C
Detection: UV, 275 nm
Injection: 2 μL
sample:
1) Caffeine
2) Theophylline
3) Theobromine
4) Huang Wei
5) Hypoxanthine
A decrease in temperature increases the resolution (degree of separation) between theophylline and theobromine, and therefore, the final method (Fig. 21) is considered to be suitable for its use.
Figure 21
Final development method: 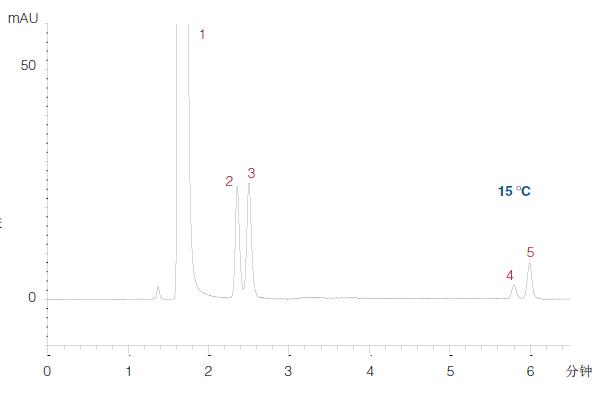
Column: ACE 5 HILIC-N, 150 x 4.6 mm
Mobile phase: A = 10 mM ammonium formate (pH 3.0) dissolved in MeCN/H2O (96:4 v/v) B = 10 mM ammonium formate (pH 3.0) dissolved in MeCN/H2O (1:1 v/v) Medium gradient: 0-100% B in 15 minutes, 100% B for 5 minutes, the next injection is maintained at the starting condition 20 minutes Flow rate: 1.5 mL/min
Temperature: 15 °C
Detection: 275 nm
Injection: 2 μL
Example 2 - Creatine and Creatinine <br> Creatine (Figure 22) is an amino acid synthesized using glycine and arginine.
It plays an important role in providing energy to cells in the body (mainly used to energize cells in the body) and forms (produces) byproduct creatinine. Determination of creatinine in the blood to determine if the kidney function is normal, where an increase in creatinine levels indicates that the kidney may not completely filter the waste. (Elevated indicates that the function of filtering waste of the kidney is reduced)
Figure 22
Structure and log P creatine and creatinine data 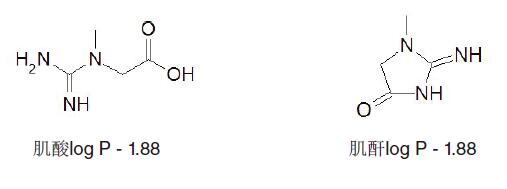
Figure 23
Isocratic screening of the ACE HILIC range using ammonium formate at pH 3.0, 4.7, and 6.0:
1) Creatinine
2) Creatine The two analytes of the three HILIC stationary phases were screened using isocratic conditions at three pH conditions.
The results showed that creatinine was properly retained in the three ACE HILIC phases, but creatine did not elute for a reasonable period of time due to excessive retention.
According to the flowchart in Fig. 17, it is more appropriate to over-retain the window to indicate the gradient (a wide period of time indicates a gradient method). 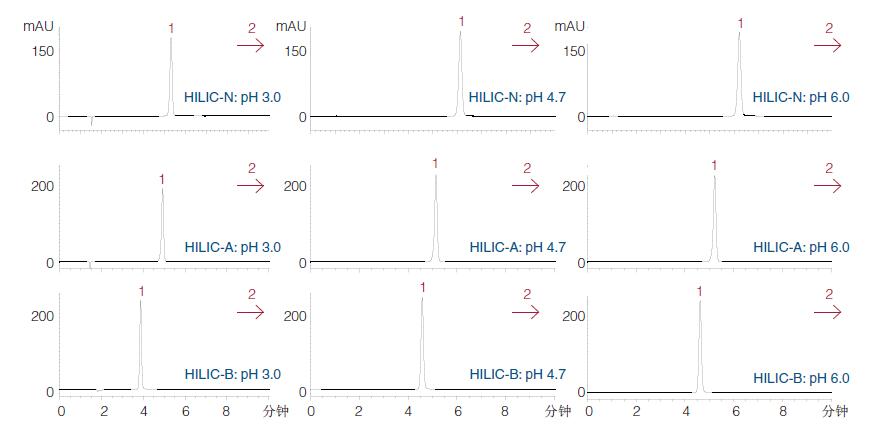
Figure 24
The final method under the ACE HILIC-A phase
ACE HILIC-A was selected at pH 3.0 for gradient analysis.
The standard gradient precipitated two target analytes in 10 minutes with high resolution (high resolution) (data not shown). Therefore, this further reduces the gradient time (in this case, the gradient run time can be further reduced), thereby shortening the overall running time.
The final method is shown in Figure 24. 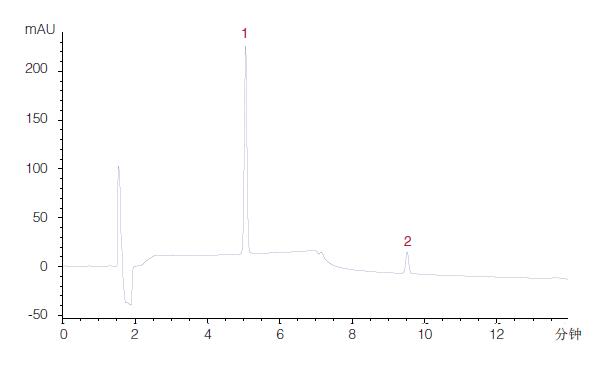
Column: 150 x 4.6 mm, 5 μm
Mobile phase:
A: 2 mM ammonium formate (pH 3.0) is dissolved in MeCN/H2O (90:10 v/v)
B: 2 mM ammonium formate (pH 3.0) dissolved in MeCN/H2O (50:50 v/v) Gradient: 5-55% B, flow rate in 10 minutes: 1.5 mL/min
Detection: 230 nm
Injection: 5 μL
sample:
1) Creatinine
2) Creatine
in conclusion
HILIC is a versatile polar analyte chromatographic method (a common chromatographic mode for polar compounds).
This method is more complicated, but if you follow simple rules, you can implement a reproducible HILIC method (the HILIC method is reproducible).
Three ACE HILIC phases (stationary phase) are designed to study selectivity during HILIC method development and provide the option to achieve the desired separation as quickly as possible.
The ACE HILIC method validation protocol (procedure) has been successfully used to develop a range of HILIC methods and should provide a structured approach to HILIC method development activities.
ACE HILIC Column Method Development Guide VI - HILIC Application Examples
ACE HILIC method development platform and working examples Figure 17 shows the flow chart of HILIC method development.