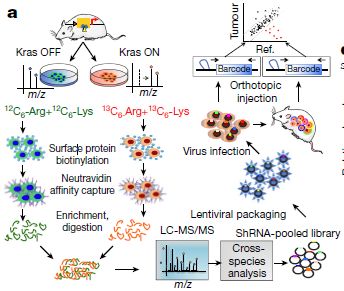
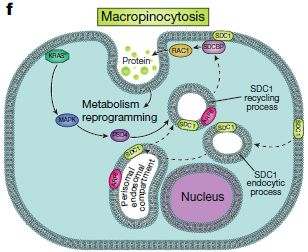
Keywords: proteome, SILAC, KRAS, cancer Professor Giulio Draetta from the University of Texas and colleagues found that a protein called syndecan-1 (SDC1) on the surface of pancreatic cancer cells responds to intracellular KRAS signaling and promotes macrophage destruction. The study was published in the latest issue of Nature. Letter | Published: 27 March 2019 Syndecan 1 is a critical mediator of macropinocytosis in pancreatic cancer Original link: https:// Tumor cells have a malignant infinite proliferative capacity that requires a lot of energy to support. Obviously, the conventional energy metabolism frequency can not meet the greedy tumor cells, it needs to destroy other normal cells by means of macrophages to meet its own energy supply. This mechanism for regulating macrophages to obtain energy is still unclear and is one of the current research hotspots. Pancreatic ductal adenocarcinoma (PDAC) is a malignant tumor of the digestive tract that is highly malignant and difficult to diagnose and treat. There is a Kras mutation in 90% of pancreatic ductal carcinoma. This mutation activates macrophages in the tumor microenvironment through an unclear mechanism and promotes endocytosis of macrophages. In order to accurately find the metabolism of pancreatic ductal adenocarcinoma, the Giulio Draetta team challenged. They constructed models of Kras open and closed states and extensively screened and studied KRAS-associated cell surface proteins through proteomics techniques in search of new targets for the treatment of pancreatic cancer. Exploring Raiders Experimental material: PDAC mouse model cells -- Kras OFF vs Kras ON 1. Quantitative proteome analysis of changes brought about by Kras signaling To investigate changes in cell surface protein expression by Kras signaling, the team compared the differences between Kras OFF and Kras ON using SILAC quantitative proteomics . A total of 221 differentially expressed proteins were obtained in this analysis, of which 196 were up-regulated and 25 were down-regulated. 2, the letter analysis analysis function and access IPA (Ingenuity pathway analysis) analysis revealed that many up-regulated expression proteins are involved in PDAC-activated biological processes, including axon-directed signaling pathways, and this result supports the notion that KRAS* is a major driver of PDAC molecular reprogramming. 3, lock attention to protein The researchers selected 110 proteins that are also present in the human PDAC cell line, as well as 37 genes that are highly expressed on the cell surface, and constructed a shRNA library to construct a mouse model. Further analysis revealed that SDC1 in the heparin sulfate family was significantly enriched on the plasma membrane during the expression of Kras. In the proteome analysis, SDC1 is also highly enriched. Therefore, in the follow-up study, the researchers locked SDC1 as a protein of interest. 4, focus on protein function verification Subsequent validation experiments showed that the deletion of SDC1 can significantly inhibit the growth of subcutaneous xenograft tumors, and also inhibit the colony formation and tumorigenicity of tumor cells. Various experimental results indicate that SDC1 on the surface of iKras* tumor cells is essential for disease progression. And it works by regulating the giant cell drink to provide energy for the growth of tumor cells. summary This study used proteomic techniques to extensively detect changes in cell surface proteins caused by changes in Kras status. Screening in differential proteins, obtaining proteins of interest, and performing a large number of functional validations, ultimately establishes a mechanism of linkage between KRAS* signaling and target molecules that drive the nutrient recovery pathway in PDAC. It also provides a strategy for subsequent tumor research to identify cancer-specific weaknesses by analyzing differences in surface protein expression. Lab Researching Microscope ,Microscope Lab,Biological Microscope,Laboratory Microscope Ningbo ProWay Optics & Electronics Co., Ltd. , https://www.proway-microtech.com