Our comprehensive electronic IQ/OQ/PM service supports the verification of new UV OQ services at any time. The compliance process for Molecular Devices microplate readers in GLP or GMP environments is now more reliable, safer and more convenient. The now-compliant, compliant, electronic IQ/OQ/PM service is Molecular Devices' unique verification solution that preserves traditional compliance service documents in a secure electronic form and is remotely accessible. Compliance verification instrument verification During each field validation process, trained Molecular Devices hardware engineers will perform calibration and electronic recording instrument operations in accordance with IQ/OQ requirements. All calibration and periodic maintenance programs automate the process of each verification through automation while ensuring the integrity of data and results analysis. Our services include: New UV OQ service Welcome to our latest UV verification service from 220 nm to 350 nm, service verification and validation - Photometric accuracy, accuracy and linearity Through installation verification (IQ) Verify that all materials necessary for installation have been received and properly installed in accordance with Molecular Devices ISO 17025 certification. Operational verification (OQ) Detect the mechanical, electrical, and optical components of each instrument and verify that the instrument will operate as intended by the manufacturer. Preventive maintenance (PM) Ensure that each instrument meets operational requirements with comprehensive multi-point inspection. By proactively addressing potential hazards, PM ensures that each instrument remains in optimal operating condition. Ensure that verification is always available The service will create a comprehensive electronic or printed report on the spot for easy verification at any time. Each report can be customized to the company's workflow, including: - Quantitative results All inspection and periodic maintenance programs are electronic services that comply with USP<1058> Analytical Instrument Qualification (AIQ) guidelines for compliance. The final report provides all the verification information necessary for verification, which is easy to audit at any time and has the following characteristics: - Electronic signature in accordance with SAFE-BioPharma Electronic reports are securely stored in a central location and support remote access, ensuring that you have all the necessary materials ready for review. core advantages - Compliance with US and EU pharmaceutical companies guidelines win-win For more maintenance plans, value-added services and support information, please visit Or contact Molecular Devices to take your lab's safety and reliability to a whole new level.
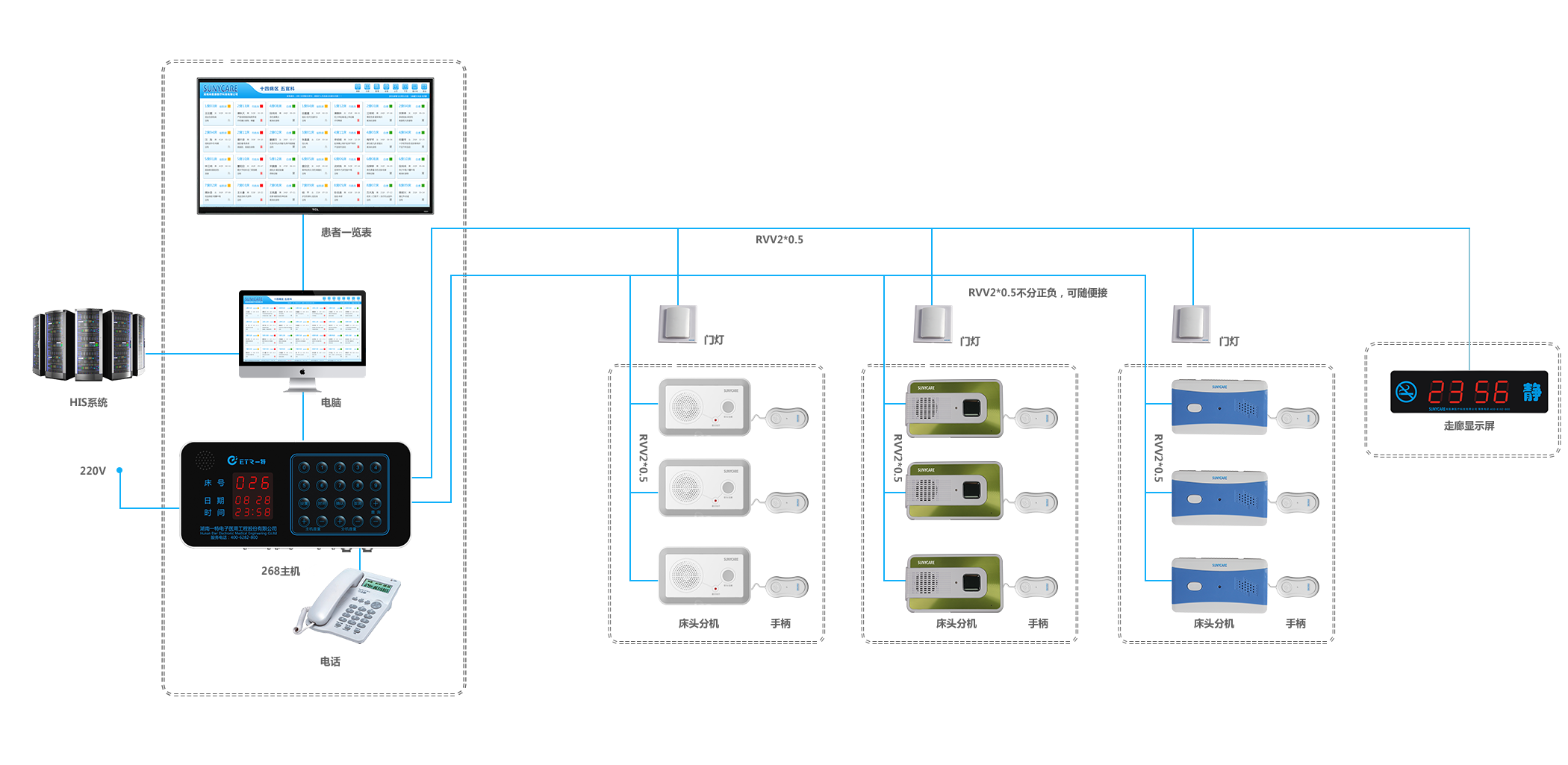
Two-wired Nurse Call System
ETR Two-Wired Nurse Call System is designed for providing the communication between patients in wards and medical personnel in nurses offices in case of any peculiar conditions in hospitals.,it has higher performance-cost ration and more simple installation than those products of the same variety.
Two-Wired Nurse Call System,Medical Nurse Call System,Calling System For Hospital,Nurse Call Station Hunan Eter Medical Co., Ltd. , https://www.eter-tech.com
- Wavelength accuracy
- stray light 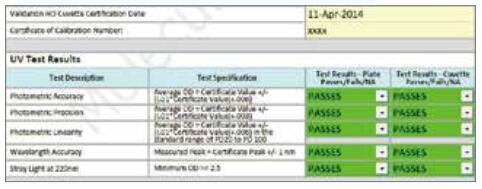
- Hardware Service Report
- Verification board quality inspection report
- Verification report
- date and time stamp
- Provides pre-verification of algorithms that comply with US Pharmacopeia guidelines
- Securely store IQ/OQ reports in electronic form, on-demand access
- Provide electronic signature with date/time stamp for compliance
- Verification of the calculation process, speeding up the verification process and improving verification accuracy