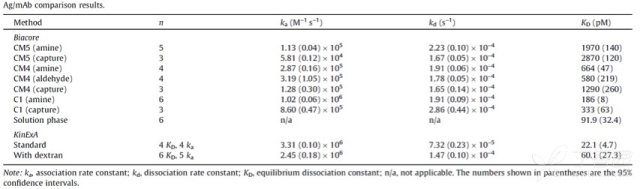
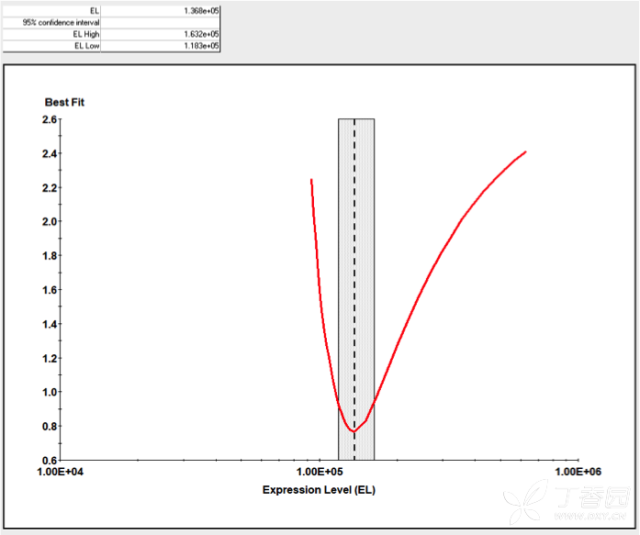
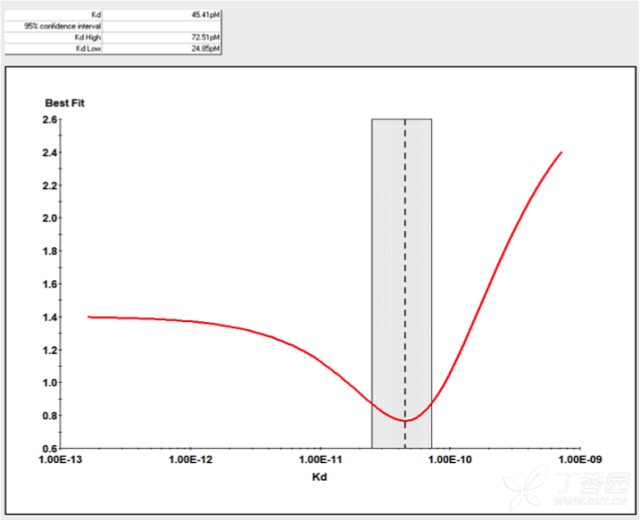
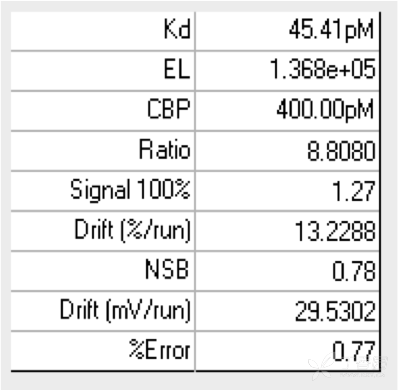
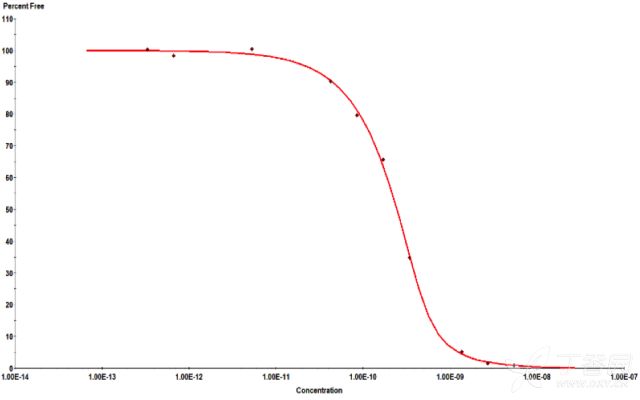
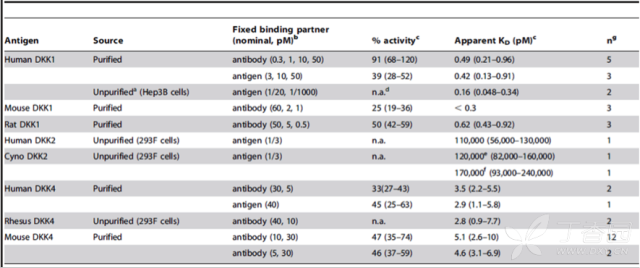
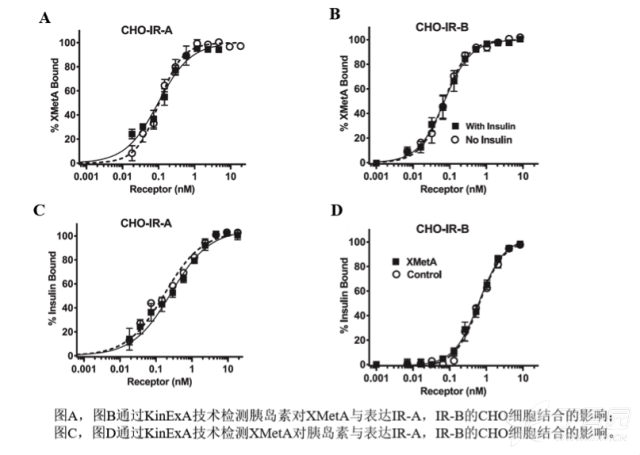
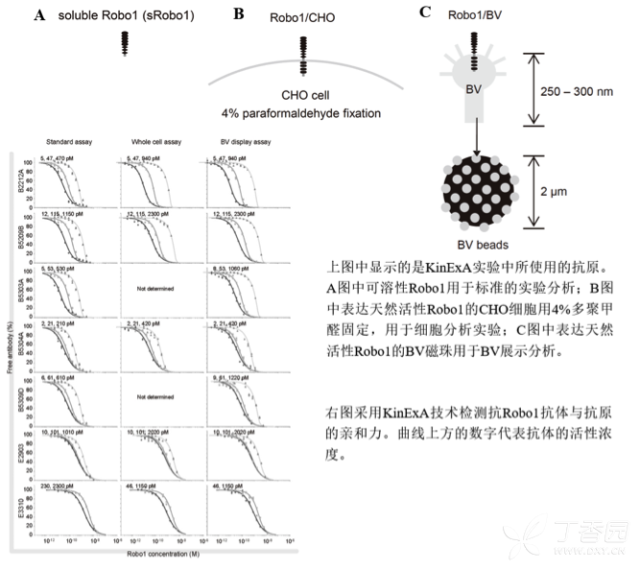
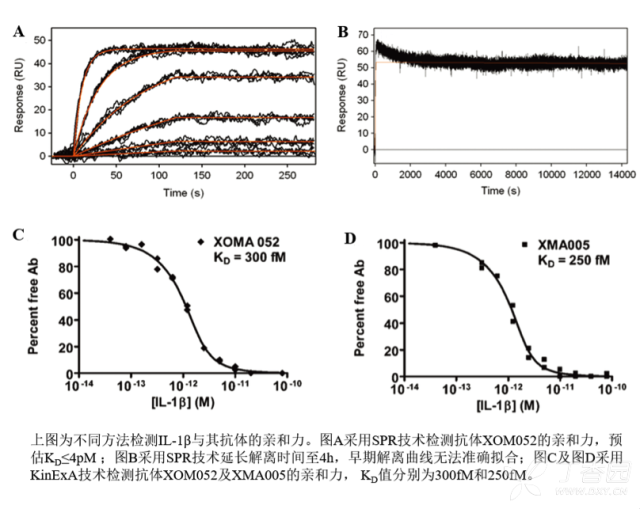
KinExA® Molecular and Cell Interaction System Introduction First, the technical background of KinExA: Sapidyne Instruments Inc. was founded in the United States in 1995 and is based on the patented unique Kinetic Exclusion Assay (KinExA) technology. In the early days of the company, Xavier University, the US Army and the Environmental Protection Agency and other research units used KinExA technology to carry out a lot of work; after decades of extensive application in the field of biopharmaceuticals, scientific research and environmental monitoring, KinExA technology has become the top pharmaceutical Companies and biotech companies, as well as many universities, independent research laboratories, and environmental monitoring agencies, study the essential tools for interaction and bioactive substance testing, and have been approved by the FDA and EMA. Second, the technical principle of KinExA: When the receptor and the ligand reach equilibrium in the reaction solution, three substances are present in the solution: the receptor, the ligand, and the receptor-ligand complex. KinExA technology captures free ligands or receptors in a very short time (<0.5s without affecting the reaction equilibrium) by coating the beads of the receptor or ligand, and then detecting the free ligand or receptor by fluorescently labeled antibodies. The amount. The detection process is as follows: Third, the difference between KinExA and SPR 1. The difference from SPR: SPR fixes one molecule on the surface of the chip, and realizes SPR detection through the chip indicating the change of the mass of the two-dimensional interaction with the solution. This has a very significant disadvantage: biomolecules immobilized on the chip may not maintain its natural activity, mass migration affects the kinetic analysis (for example, the flow rate will affect the experimental results), the detected molecule has a lower molecular weight limit, and is very large. The molecular or biological structure has an upper limit on the molecular weight, the sample needs to be purified, and the intact cells cannot be detected. Conversely, KinExA analyzes three-dimensional and free-state interactions, does not immobilize any molecules, does not affect balance, has no mass migration limitations, can detect unpurified samples and intact cells; therefore, a wide range of biomolecules, Both biological structures and intact cells can be analyzed flexibly. 2. Comparison with SPR technology: In order to characterize therapeutic monoclonal antibody candidate molecules, the researchers used different types of chips to obtain the same group of monoclonal antibody-antigen 53 data from the Biacore system. Compared with the KinExA experimental data, the affinity and kinetics were found. The data is related to the type of chip used. The negatively charged CM5, CM4 and CM1 chips have a detrimental effect on Biacore's kinetic data. To test this hypothesis, the authors accurately calculated the affinity and kinetic parameters of antibodies and antigens by Biacore liquid phase experiments, KinExA equilibrium titration and KinExA kinetic experiments. The results show that as the negative charge on the surface of the chip decreases, the affinity and kinetic parameters are closer to those obtained in the liquid phase experiment. Possible causes: (1) steric hindrance between the negatively charged dextran chip and the antibody affects the binding of the antigen; (2) negatively charged antigen and negatively charged electrostatic repulsion on the surface of the chip. The results in the table indicate that for the Biacore technology, different fixation methods (amino coupling, capture) and different chips have a significant impact on the experimental results. With the KinExA technology, the addition of dextran to the solution had no significant effect on the results. Drake AW, et al. 2012. Biacore surface matrix effects on the binding kinetics and affinity of an antigen/antibody complex. Anal Biochem. 429(1): 58-69. Fourth, the application of KinExA: Application 1. Application of KinExA in CAR-T cell therapy Due to a variety of factors, autologous CAR-T cell therapy products may not be able to perform validation of all items before they are applied to patients, so process validation is very important. Process research and verification must be based on the actual conditions of the process to set the corresponding verification parameters, CAR-T cell product technology research is constantly developing, so far, the industry has not reached a consensus on which process is best. Therefore, different levels of process validation studies can be carried out during product development and early clinical trials. After process validation, key process control parameters and standards should be set at key process steps to improve production consistency of CAR-T cell products. Quality control studies and testing programs for CAR-T cell therapy products should generally include: cell number and survival, cell phenotype, CAR positive rate detection, biological efficacy test, sterility test, mycoplasma, pyrogen/endotoxin Detection, detection of viral vector copy number and integration in CAR-T cells. If it can detect the affinity of CAR-T cells with antigen molecules and the expression of antibodies on CAR-T cells, it will undoubtedly improve the quality control standards of CAR-T cells. In addition to KinExA technology, there is no other particularly effective way to detect the affinity between intact cells and molecules, and it is impossible to judge the expression of molecules on cells. KinExA technology detects cell-to-molecular affinity and calculates the amount of molecule expression on a cell. The detection principle is as follows. First, the gradient-diluted cells are incubated with a constant concentration of molecules to achieve equilibrium. After centrifugation, the supernatant is collected (only free molecules are present in the supernatant), and then passed through the pre-coated The beads captured the free molecules, and the amount of free molecules was detected by the fluorescent-labeled antibody. After the corresponding values ​​were detected by the detector, the data was analyzed using the KinExA system software. The figure below shows the results of a well-known CAR-T company in China testing the interaction between CAR-T cells and antigen molecules through KinExA technology. The results showed that the affinity Kd of CAR-T cells to antigen was 45.41pM, the expression level of CAR on T cells was 1.368e+5, and the 95% confidence intervals of Kd and EL were narrow. The data were very accurate and credible. Application 2, KinExA application in high affinity testing For the anti-tumor drug market, the most mature field of precision medical care is anti-tumor drugs represented by targeted drugs. Because monoclonal antibody anticancer drugs have fewer side effects and better targeting, monoclonal antibody drugs will continue to be the most important field for the development of anticancer drugs. A class of monoclonal antibodies targeting PD-1 exhibits a high affinity, and conventional interaction detection systems such as SPR, BLI, ITC, etc. cannot detect high affinity (pM level) due to limitations of their own principles. KinExA technology is different from conventional interaction detection systems in that it can accurately and efficiently detect high affinity (fM level). The following table is an article published by the two companies Pfizer and Rinat for the high affinity detection range of KinExA. Bee C., et al. 2012. Exploring the dynamic range of the kinetic exclusion assay in characterizing antigen-antibody interactions. PLOS ONE 7(4): e36261. V. Case analysis Case 1: Detection of intact cell interaction BACKGROUND: The monoclonal antibody XMetA is an agonist of the insulin receptor (IR) allosteric component, which activates the metabolic Akt kinase signaling pathway and has little effect on the mitotic extracellular signal-regulated kinase (ERK) signaling pathway. To investigate the nature of this selective signaling pathway, the authors validated the effect of XMetA on the specific phosphorylation and activation of IR, Akt and ERK in CHO cells. Objective: To detect intact cell affinity. METHODS: The researchers compared different concentrations of CHO cells expressing short-chain (IR-A) and long-chain (IR-B) insulin receptors to XMetA, and obtained free XMetA by centrifugation, and the affinity was detected by a KinExA instrument. In addition, the authors used the same strategy to detect the affinity of insulin with CHO cell surface IR-A, IR-B using the KinExA instrument. Conclusion: The affinity of XMetA with IR-A subtype is 55±16pM, and the affinity with IR-B subtype is 50±11pM. In addition, in the control antibody group, the affinity of insulin to IR-A subtype was 156 ± 14 pM; in the XMetA group, the affinity of insulin and IR-A subtype was 216 ± 100 pM; in the control antibody group, insulin and IR-B sub The affinity of the type was 221 ± 28 pM; in the XMetA group, the affinity of insulin to the IR-B subtype was 277 ± 112 pM. The data also demonstrates that the binding of XMetA to the IR subtype is not associated with insulin. Bedinger, D., et al. 2015. Differential pathway coupling of activated insulin receptor drives signaling Selectivity by XmetA, an allosteric partial agonist antibody. J Pharmacol Exp Ther 353(1): 35-43. Case 2: Detection of unpurified samples of cells and supernatant BACKGROUND: The reliable assessment of the affinity of monoclonal antibodies (mAbs) in vivo with membrane proteins is a major problem in cancer therapy. In the BV display system, membrane proteins can be displayed on the surface of the virus in a natural state. OBJECTIVE: To detect the affinity of unpurified samples in cells and supernatants. METHODS: Based on KinExA technology, the researchers described a simple and highly sensitive method for the evaluation of monoclonal antibodies in combination with a baculovirus (BV) membrane protein display system. Conclusion: The liver cancer antigen Robo1 displayed on the surface of BV is adsorbed onto magnetic beads (BV beads), and its KD value (~10pM) is consistent with the whole cell analysis method (R2=0.998), indicating that the method based on KinExA technology provides a cell surface. The monoclonal antibody affinity of the protein is accurately assessed. Kusano-Arai 0., et al. 2016. Kinetic exclusion assay of monoclonal antibody affinity to the membrane protein Roundabout 1 displayed on baculovirus. Anal Biochem. Case 3: High affinity testing BACKGROUND: Interleukin-1β (IL-1β) is an effective mediator of inflammatory responses and plays a regulatory role in the differentiation of many lymphocytes. In some inflammatory and autoimmune diseases, serum IL-1β levels are associated with the development and severity of the disease. The mechanism of IL-1β in some diseases has been confirmed by clinical trials and approved by the FDA. Purpose: High affinity detection and verification. Methods: Anti-IL-1β antibody XOM052 was designed and its affinity with IL-1β was ≤4pM. In addition, Protein A was captured by Protein A and dissociated for 10 min. It was found that the time was not enough to dissociate the antibody antigen, and the dissociation time was extended to 4 h. The early dissociation could not be accurately fitted. It is presumed that the IL-1β antibody was derived from The effect of dissociation on Protein A on the experiment. In order to calculate the affinity more accurately, the author changed to KinExA and analyzed the affinity to 300fM. Conclusion: KinExA technology has an irreplaceable advantage for high-affinity detection. Owyang AM, et al. 2011. XOMA 052, a potent, high-affinity monoclonal antibody for the treatment of IL-1B-mediated diseases. mAbs 3(1): 49-60. Six, KinExA reference literature, please contact us to obtain. Teacher wants to call Feed Additives Fruit Powder,Echinacea Purpurea Extract,Saccharomyces Cerevisiae Dextran,Polyphenols Echinacea Purpurea Extract Fufeng Sinuote Biotechnology Co.,Ltd. , https://www.ffsinuoteplant.com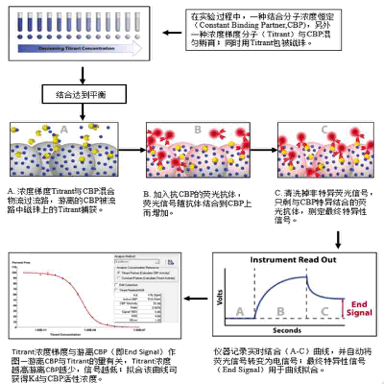

Shanghai Shuangmo Biotechnology Co., Ltd.
QQ