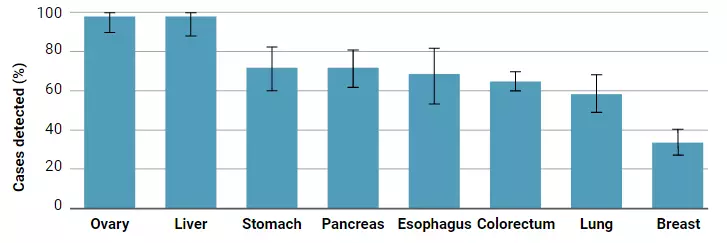
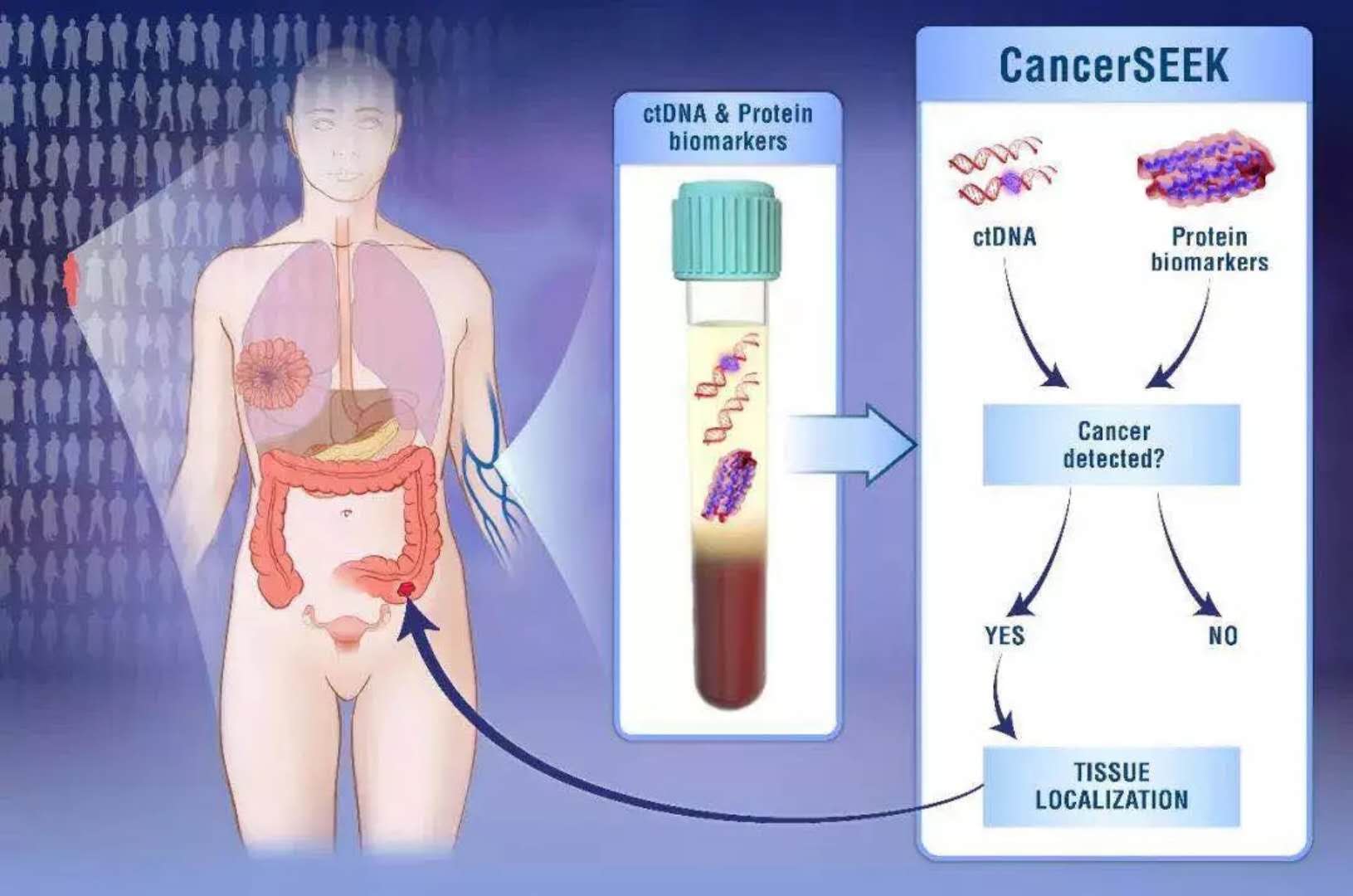
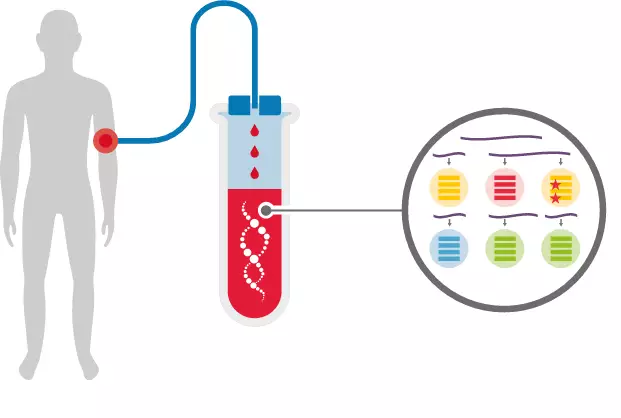
"Science" published a major breakthrough in early detection of cancer, quickly and low-cost detection of 8 common cancers, terminal illness ushered in prediction January 22, 2018 Source: DeepTech Deep Technology "Death" cancer has always been a sensation. People have been exploring ways to detect cancer before it can be abnormal. Nowadays, you can judge you by drawing blood. A convenient method of detecting whether a body is lurking with a tumor and being able to locate a latent tumor is about to become a reality, and it is inexpensive and may cost you only $500. Although the proportion of tumor DNA in the blood is less than 0.1%, scientists still hope to achieve a non-invasive diagnosis of cancer through this tiny signal. On January 18th, a research team led by Nickolas Papadopoulos, an oncologist and director of the Department of Transformed Genetics at Johns Hopkins Kimmel Cancer Center, published a paper in Science. A novel blood test method based on a combination analysis of ctDNA (circulating tumor DNA) and protein biomarkers. This test method correctly detected the 8 most common cancers, with a sensitivity of 98% for ovarian cancer. (Sensitivity: Probability of correct detection of cancer) Figure çµæ•åº¦ Sensitivity of 8 cancer detections (from left to right: ovarian cancer, liver cancer, stomach cancer, pancreatic cancer, esophageal cancer, colon cancer, lung cancer and breast cancer) This new method of detection will likely be a major advancement in the field of liquid biopsy technology, which allows your blood sample to detect cancer before you feel unwell or before you find a tumor. The significance of this is very significant, because early cancer can usually be cured before it spreads. Liquid biopsy technology was also named "Breakthrough Technologies 2015" by MIT Technology Review, and the reason for the list is "Fast and Simple Cancer Blood Test Method." This new test method is called "CancerSEEK". When designing for CancerSEEK, the team determined the optimal number of DNA bases used in CancerSEEK testing by the principle of diminishing returns. Later, they applied the Safe-Sequencing system (Safe-SeqS) developed by JHU scientists to effectively utilize a small amount of ctDNA in plasma. In addition, they also divide the total DNA recovered from plasma into multiple aliquots for independent sequencing, reducing the number of DNA molecules in each sequencing well, but the detection ratio of mutant molecules in each sequencing well is increased. This means that the mutants in the sample are more easily found. The above is the detection part of CancerSEEK on ctDNA, and the other part is the detection of protein biomarkers. Since most early tumors did not release ctDNA, the team studied protein biomarkers in plasma samples and found that eight proteins differentiate cancer patients from healthy controls on a single immunoassay platform. ※ 8 kinds of proteins: cancer antigen 125, carcinoembryonic antigen, cancer antigen 19-9, prolactin, hepatocyte growth factor, osteopontin, peroxidase and metalloproteinase 1 tissue inhibitor. Finally, the researchers used this method to detect blood samples from 1005 people who were previously diagnosed with cancer in the ovaries, liver, stomach, pancreas, esophagus, colorectum, lungs, or breast. The researchers aimed to find proteins associated with the eight cancers mentioned above, as well as related 16 gene mutations. The researchers found that this new test has the best effect on the diagnosis of ovarian cancer, which identified 98% of ovarian cancer cases and correctly identified one-third of breast cancer cases and approximately 70%. Cases of pancreatic cancer (pancreatic cancer is now very difficult to detect). Finally, the researchers tested the specificity of this test. They performed this test on 812 people who were clearly healthy, and only 7 of them were positive. It can be seen that its specificity is still very high. Figure 丨 CancerSEEK detection As mentioned earlier, this test method can also determine the part of the body where the cancer is located. This is because researchers have trained a machine learning algorithm to find out the correlation between the blood sample information and the location of the cancer. In the test, the correct recognition rate of this algorithm reached 83%. Although this new test method has not yet been put into commercial use, it will be used to test 50,000 women of retirement age who do not have a history of cancer. This is actually part of a $50 million, five-year research project in partnership with the Geisinger Health System. “We believe this new test method will be accepted by the public in the future,†said Nickolas Papadopoulos. "This test method does not hurt you because it is aimed at your blood sample." Nickolas Papadopoulos, Oncologist and Director of Translational Genetics, Canmore Cancer Research Center, Johns Hopkins University “I think we will eventually find a way to detect cancer before it is discovered or if it is abnormal,†commented Len Lichtenfeld, deputy chief medical officer of the American Cancer Society. However, Lichtenfeld also reminded that researchers have to improve the specificity of blood testing methods, otherwise it will bring psychological shadow to the original healthy person if it is wrong. Of course, this latest research is just one of many “liquid biopsies†tests being developed. The so-called "liquid biopsy" is a technique for detecting and acquiring relevant disease information by using human body fluid such as blood or urine as a sample source. As a branch of in vitro diagnosis, an important application direction of liquid biopsy is cancer detection. Figure 丨 liquid biopsy brief process However, most published studies, including this study, focus on measuring and monitoring advanced tumor stages. A small number of liquid biopsies have even been approved for the matching of tumors with targeted drugs. However, people dream of developing a simple blood test that can be used to accurately diagnose whether a healthy person has a solid tumor. The scarcity of circulating cancer biomarkers (whether in mass or quantity, circulating tumor DNA accounting for less than 0.1% of blood) has made this desire stagnant for decades. But now, sensitive assays and computing platforms are driving the discovery of biomarkers and using better methods to measure these markers, thus attracting a pool of well-funded start-ups into the field. For example, in 2016, Illumina, the world's largest sequencing company, split a company called Grail. The company's mission is to “find cancer in the early stages of being cured.†Last year, this ambitious goal was supported by a $1.2 billion venture capital fund to fund large-scale, population-based clinical trials. Research and optimize its sensitive sequencing technology. The reason why the business community is so optimistic about liquid biopsy technology is that this technology does not damage the body of the subject to some extent like the physical and chemical detection method. Grail has not yet released any actual data, and its website only advertises comments posted last year in Cell magazine. And its main competitor in the field, machine learning startup Freenome has not released any data yet. In March last year, the company, which was founded only three years by Andreessen Horowitz, received a $65 million first-round investment. Freenome's gaze is not limited to the "genetic debris" left by tumor cells - it also captures other disease characteristics in the blood, such as changes made by the immune system in response to the tumor's microenvironment. Of course, Freenome is not sure how this test works. "The card will be finally brighter." Vijay Pande, the partner of the investment company's biological assets and partner of Anderson, said: "The publication shows that your attention is not on the founding company." Even so, he really hopes that Freenome is at first Before entering the market, you can publish articles in peer-reviewed journals. However, the person who is sick may be anyone. To assess the effectiveness of these blood tests, thousands of patients are subject to examination – and then researchers wait for some of them to have cancer. This is not only the only way to detect its predictive power, but also a way to determine if a patient's prognosis is improved. Currently, available non-invasive screening tests (mammography for breast cancer, protein measurement tests for prostate cancer) have their own limitations. Misdiagnosis not only causes patients to waste time and money on treatment, but also burdens unnecessary psychological stress. Geoff Oxnard, a thoracic oncologist at the Dana-Farber Cancer Institute and a professor at Harvard Medical School, says liquid biopsies are likely to be subject to the same controversy. He often uses the single-gene liquid biopsy developed by the Dana Faber Institute to determine which drugs are the best choice for patients with lung cancer. But, one day, will early cancer screening become part of a routine checkup by a doctor? "No," he said. “I think these tests will help us better understand the risk of cancer in some patients. Some of the family members of these patients already have cancer or have already found some disease in the scan. But I don’t think we can Liquid biopsy is seen as a panacea for cancer diagnosis. In the final analysis, it is just a shortcut." Tudana Faber Cancer Institute However, Oxnard pointed out that the Papadopoulos experiment is an important step forward. First, determine where the tumor is occurring. This is a major limitation of liquid biopsy. Yes, you found cancer, but what are you going to do next? Where is the tumor? Most mutations do not display location information. But by measuring 31 additional proteins in its machine learning model, Johns Hopkins' research team was able to correctly identify 80% of cancers in patients with colorectal, pancreatic, and ovarian cancers on the first attempt. organization. Also, consider the cost. Papadopoulos estimates that the trial may be commercialized at around $500. Existing screening tests only look for a single gene, and cancer detection methods that rely on ultra-deep sequencing may increase the cost of existing screening trials. “This is significant in its field and ensures that these analyses can become clinically realistic.†Victor Velculescu, a historian at Johns Hopkins University and a colleague of Papadopoulos. Say. He also developed liquid biopsy technology, but did not participate in the research of Science. Victor Velculescu, a historian at Johns Hopkins University Since turning Baltimore into their own liquid biopsy center, the two of them have started a enclosure friendly match. Recently, two researchers have created their own diagnostic company to further develop their early detection technology platform. Earlier this month, Wilkescu's private genome diagnostics company received a second round of financing from pharmaceutical giant Bristol-Myers Squibb for $75 million. This has enabled its total financing to reach $99 million, which can be compared with some well-known peers in the same field, adding more variables to market competition. Regardless of the outcome, the patient is the ultimate beneficiary. “Even if there are still 50% of cancers, we still have no way to screen them out, but 50% of patients can still be treated in the first phase, and they still have a chance,†Papadopoulos said. "It's not necessarily perfect, but it still saves lives." reference: Http:// Https:// More Chinese Seasonings,White Sesame Oil,Organic Black Sesame Seeds Paste,Organic Roasted White Sesame Seeds,Pure Organic Black Sesame Seeds Paste Chinese Seasoning (Shandong) Trading Co.,Ltd , https://www.zt-trading.com