Wiggle wire lock is designed to be used with poly lock base cap- sold separately. Greenhouse wiggle wire and base care are an effective method of attaching your poly film roof and or shade cloth. The greenhouse wiggle wire lock will hold form 4 mil to 20 mil thickness of material. Use one wire to hold the roof in place and then add a second wire to hold your shade cloth. When it is time to remove the shade cloth, you will not disturb the roof plastic. Greenhouse Wiggle Wire,Greenhouse Steel Wire,Greenhouse Spring Wire JIANGSU SKYPLAN GREENHOUSE TECHNOLOGY CO.,LTD , https://www.engreenhouse.com
Waters Corporation, Milford, MA, USA
Program Benefits â– Use XP columns to improve the time-consuming USP US Pharmacopoeia organic impurity analysis method for faster analysis and reduced solvent usage while still complying with the US Pharmacopoeia <621> guidelines.
â– Reduced sample run time by 80%, increasing productivity.
â– Reduce solvent usage by 90%.
Waters offers solutions
ACQUITY UPLC ® H-Class System
Alliance ® HPLC System
XSelectTM CSHTM C 18 column
Empower ® 3 software
eXtended Performance [XP] 2.5 μm column TruViewTM LCMS certified maximum recovery vial
Key words <br> USP method, tioconazole, ACQUITY UPLC Columns Calculator, Waters reverse phase column selection table, generic drugs
INTRODUCTION <br>The pharmaceutical companies around the world need to analyze the organic impurities in generic drugs in their daily work. Organic impurity analysis using older instrumentation and column technology is time consuming and expensive because of the large amount of solvent required for long periods of time. However, organic impurity analysis becomes more efficient by using significantly improved instrumentation and column technology. The 2.5μm particle size eXtended Performance (XP) column is designed for high performance liquid chromatography and ultra high performance liquid chromatography. This column is ideal for improving the US Pharmacopoeia method because it enables chromatographers to realize the benefits of smaller particle size and low-diffusion systems while meeting the requirements of the US Pharmacopoeia <621> Chromatographic Analysis Guide. The <621> chapter lists the extent of the allowed method changes.
Thiconazole is an imidazole antifungal compound used to treat yeast infections. The method to be converted is the analysis method 2 of the organic impurities of tioconazole. The organic impurity analysis method is used to determine whether impurities and their contents are present in the sample. The XP column method was scaled from the USP method of the column scale originally on the HPLC system to HPLC and UPLC instruments. Improvements to current USP methods using XP columns on HPLC instruments can reduce run time and increase sample throughput in routine analytical laboratories. Using an XP column on a UPLC system can further reduce run time and reduce solvent usage compared to HPLC, saving total cost.
Experimental condition
Alliance 2695 HPLC chromatographic conditions <br> Mobile phase: 44:40:28 acetonitrile / methanol / aqueous ammonium hydroxide was added 2 mL separation mode: Isocratic Detection wavelength: 219 nm
Column (L1): XSelect CSH C 18 , 4.6 x 250 mm, 5 μm,
Part number; XSelect CSH C 18 XP, 4.6 x 150 mm, 2.5 μm,
Part number; XSelect CSH C 18 XP, 4.6 x 100 mm, 2.5 μm,
Part number column temperature: 25 °C
Needle washing solution: 95:5 acetonitrile/water sample cleaning solution: 95:5 water/acetonitrile seal rinse: 50:50 methanol/water flow rate: Adjust the injection volume according to the method: Adjust according to the method
ACQUITY UPLC H-Class <br> chromatographic conditions Mobile phase: 44:40:28 acetonitrile / methanol / aqueous ammonium hydroxide was added 2 mL separation mode: Isocratic Detection wavelength: 219 nm
Column (L1): XSelect CSH C 18 XP, 4.6 x 150 mm, 2.5 μm,
Part number; XSelect CSH C1 8 XP, 4.6 x 100 mm, 2.5 μm,
Part number; XSelect CSH C1 8 XP, 2.1 x 150 mm, 2.5 μm,
Part number column temperature: 25 ° C
Needle Wash: 95:5 Acetonitrile/Water Sample Wash: 95:5 Water/Acetonitrile Seal Wash: 50:50 Methanol/Water Flow Rate: Adjust the injection volume according to the method: Adjust the data management according to the method: Empower 3 software
Sample description
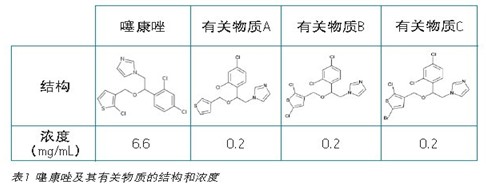
Tioconazole results and discussion <br> worldwide pharmaceutical companies need to prepare for conventional methods for routine analysis. This application note compares the separation of tioconazole and its related substances A, B, and C on several different chromatographic columns using the organic impurity analysis method specified in the US Pharmacopoeia monograph. Since many impurities of tioconazole lack practical availability, the terconazole-related substances A, B, and C are used as low-concentration impurity standards. The organic impurity analysis method listed in the US Pharmacopoeia is used to analyze complex sample formulations. Effective separation of multiple components in a sample usually requires the use of longer columns. Long columns with larger particle sizes (≥3.5 μm) will increase run time and increase solvent usage. For example, the analysis of tioconazole organic impurities in the original US Pharmacopoeia required the use of a 4.6 x 250 mm, 5 μm column with a separation time of up to 30 minutes, requiring 30 mL of solvent per sample. However, using a 2.5μm particle size eXtended Performance (XP) column, you can reduce your run time while still meeting the assessment requirements. As the run time is reduced, the sample throughput is increased and the solvent required for each analysis is reduced, reducing the total cost. The current US Pharmacopoeia Chapter <621> Chromatographic Analysis Guide specifies the extent of the method variation allowed. These allowable variations include ±70% column length change, -50% particle size change, and ±50% flow rate change. 1 US Pharmacopoeia requires that the separation between substances B and C should be 1.5, this application note proves that the method conversion between different columns and different chromatographic systems fully meets the harsh requirements for these two difficult-to-separate compounds. Claim.
Using XP HPLC instrument on a color spectrum analysis of organic impurities in the column to carry out analysis of organic impurities <br> tioconazole L1 requires the use of special column for separation column lists the LiChrosorb RP-182. Referring to the Waters Reversed Phase Column Selection Table, this article uses a more advanced XSelect CSH C18 stationary phase column. The XSelect CSH C 18 column was chosen because it is similar to the listed columns and offers a wide range of sizes and particle sizes for HPLC UPLC instruments. This article first used a XSelect CSH C 18 , 4.6 x 250 mm, 5 μm column to run the US Pharmacopoeia method on an Alliance HPLC system at a flow rate of 1.0 mL/min. As shown in Table 2, this separation meets the assessment criteria. The total run time of this separation is 30 minutes, and it will face the double challenge of time and cost management when analyzing samples in batches. If the original USP method is used, only 16 samples can be analyzed in one working day of 8 hours, consuming 480 mL of solvent. By using an XP column, 80 samples can be analyzed within the same 8-hour working day, and only 240 mL of solvent is used, significantly increasing sample throughput and reducing operating costs.
The standard method of using 2.5μm XP columns on different systems is versatile and still meets the requirements of the US Pharmacopoeia <621> guidelines, as shown in Figure 1. The XP column is a 2.5-μm particle HPLC and UPLC column that is efficiently packed and can withstand the high pressures of the UHPLC system, allowing XP columns to be used on both HPLC and UPLC instruments. 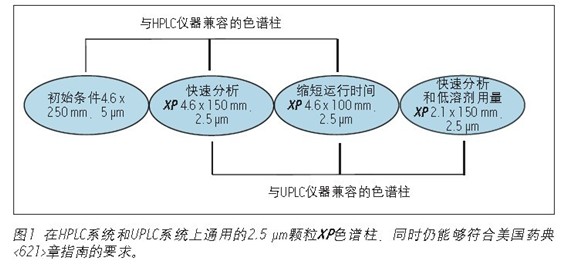
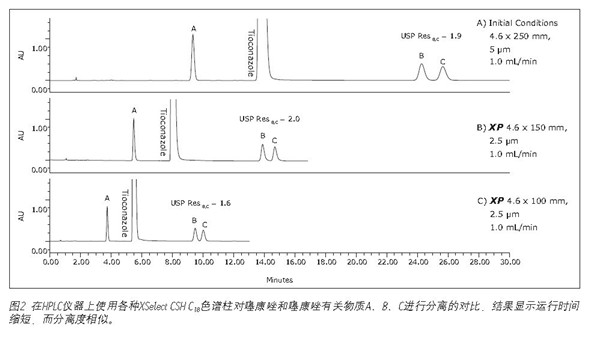
This was followed by a shorter 4.6 x 100 mm, 2.5 μm XP column to demonstrate that a faster separation was achieved while maintaining acceptable resolution. The reduction in run time is especially useful for organic impurity analysis due to the additional separation complexity, which generally has a longer run time than other methods. An important issue to note is that shorter columns with lower separation capabilities (L/dp 40,000) are not always available. For example, it may be necessary to maintain the original separation capacity in the case where the excipient and impurity elution times are very close. Figure 2C shows that when using a 4.6 x 100 mm, 2.5μm XP column, the run time is reduced by 57% compared to the initial conditions and still meets all of the evaluation criteria, as shown in Figure 2. In this case, a decrease in L/dp from 50,000 (initial conditions) to 40,000 results in a 15% reduction in the separation between the substances B and C; however, the resolution is still satisfactory, depending on the complexity of the original separation.
UPLC instrument used on XP chromatography column to carry out the analysis of organic impurities <br> shown in Figure 1, and by simultaneously using XP Columns ACQUITY UPLC Columns Calculator, the method may transition from Alliance HPLC system to ACQUITY UPLC H -Class system. Newer instruments, such as the ACQUITY UPLC H-Class system, enable faster, more efficient separation due to its high back pressure tolerance, faster balance between injections, and significantly reduced system volume and diffusion. To compare the separation capabilities between the HPLC and UPLC systems, the organic impurity analysis method using the 4.6 x 150 mm, 2.5 μm particle XP column shown in Figure 2B was re-run on the ACQUITY UPLC H-Class system, such as Figure 3A shows. Changes from the instrument itself—from HPLC to UPLC—will increase the separation between the B and C peaks by 5% and the run time by 12%, as shown in Tables 2 and 3. The increase in resolution is attributed to the low system volume and low diffusion of the UPLC system, as both properties improve peak shape. 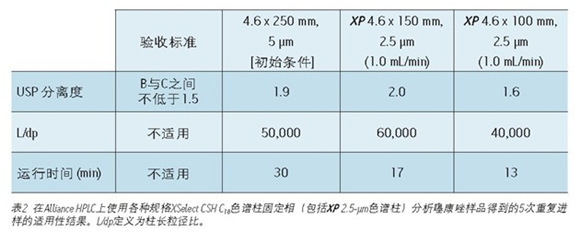
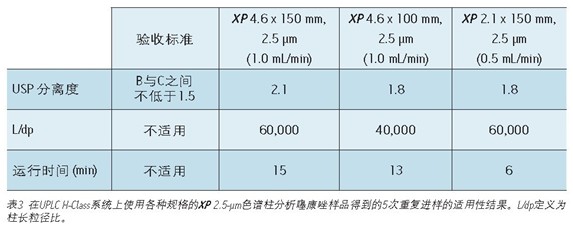
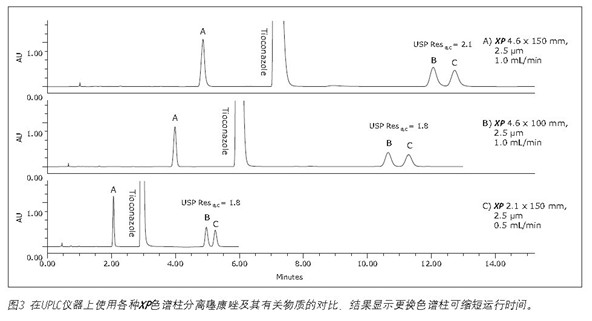
Finally, convert the standard method to a 2.1 x 150 mm 2.5 μm XP column. The results of this column show that by reducing the internal diameter of the column, while maintaining the same resolution, the run time can be further reduced and the amount of solvent is greatly reduced. The flow rate for this column is 0.42 mL/min, calculated from the ACQUITY UPLC Column Calculator. However, this flow rate is beyond the requirements of the US Pharmacopoeia Chapter <621>, so the test uses a specified flow rate of 0.5 mL/min. The resulting chromatogram (shown in Figure 3C) shows that as shown in Table 3, the run time is reduced by 80% compared to the original conditions, and the suitability requirements are still easily achieved. In addition, analysis by simply reducing the internal diameter of the column is 63% faster than using a 4.6 x 150 mm XP column, as shown in Figure 3A. Finally, by using a 2.1 x 150 mm XP column, solvent usage is reduced by 90% compared to the original standard method, resulting in significant cost savings. When the flow rate was adjusted to remain within the limits specified in the US Pharmacopoeia <621> guidelines, the resolution of the B and C peaks decreased from 1.9 to 1.8, but still met the assessment criteria.
Conclusions <br>The eXtended Performance [XP] 2.5 μm column is used on existing HPLC systems for time-consuming and cost-effective analysis of organic impurities, reducing run time and reducing compared to the original USP method The amount of solvent used was 57%. By combining XP columns with UPLC instruments, run time can be reduced by 80% and solvent usage by 90%. The utility of an XP column that can be run on both an HPLC instrument and a UPLC instrument can be used to improve the US Pharmacopoeia method while following the current US Pharmacopoeia <621> guidelines. In a conventional analytical laboratory, the use of a modified USP method with smaller particle size columns can save significant time and operating costs.
references
1. USP General Chapter <621>, USP35-NF30, 258. The United States Pharmacopeial Convention, official from August 1, 2012.
2. USP Monograph. Tioconazole, USP35-NF30, 4875. The United States Pharmacopeial Convention, official from August 1, 2012.
3. Jones MD, Alden P, Fountain KJ, Aubin A. Implementation of Methods Translation between Liquid Chromatography Instrumentation. Waters Application Note 720003721en. 2010 Sept.
Universal Base (WIG2000)- used on the curved surface, i.e. the arch for attaching the end wall covering.
Standard Base (WIG1000) - used along the straight lengths of your greenhouse.
Improvement of USP Thiconazole Organic Impurity Analysis Method Using eXtended Performance (XP) Column
Kenneth D. Berthelette, Mia Summers and Kenneth J. Fountain
The tioconazole samples were prepared at a concentration as described in Table 1 using 100% methanol. Transfer the sample to a TruView Max Recycling vial for injection (part number CV).
The standard method of this document was first converted from an initial 4.6 x 250 mm, 5 μm column to a 4.6 x 150 mm, 2.5 μm XP column to demonstrate that shorter column sizes can be used to reduce run time. The use of smaller particle sizes also improves the separation capacity, which can be predicted by the ratio of column length to particle size (L/dp). In this example, the L/dp is increased from 50,000 (initial conditions) to 60,000 (4.6 x 150 mm XP columns). The optimum flow rate for this XP column is 2.0 mL/min3, calculated from the ACQUITY UPLC Column Calculator. However, this flow rate is outside the scope of the US Pharmacopoeia chapter <621> guidelines. Therefore, a flow rate of 1.0 mL/min is used to ensure compliance with the US Pharmacopoeia guidelines and to accommodate the back pressure limitations of the HPLC system. The comparison of tioconazole and its related substances on the original column on a 4.6 x 150 mm XP column is shown in Figure 2A-B. The 4.6 x 150 mm XP column reduces run time by 43% and resolution by 5%, as shown in Figure 2.
To further illustrate the advantages of the UPLC instrument, a 4.6 x 100 mm XP column was used for separation on a UPLC system as shown in Figure 3B. This separation operation increased the resolution between the peaks of B and C from 1.6 (see Table 2) when using the HPLC system to 1.8 when using the UPLC system (see Table 3). Using a 4.6 x 100 mm XP column on a UPLC system gave the same resolution as the original method on the HPLC system, but 57% faster than the original method.